PD-1 inhibitor-ezabenlimab
A humanized PD-1-targeting monoclonal antibody that blocks the interaction between PD-1 and its ligands
A PD-1 inhibitor-ezabenlimab
ezabenlimab (PD-1 Inhibitor) Proposed MoA
| Status | Phase 2 |
|---|
Molecule
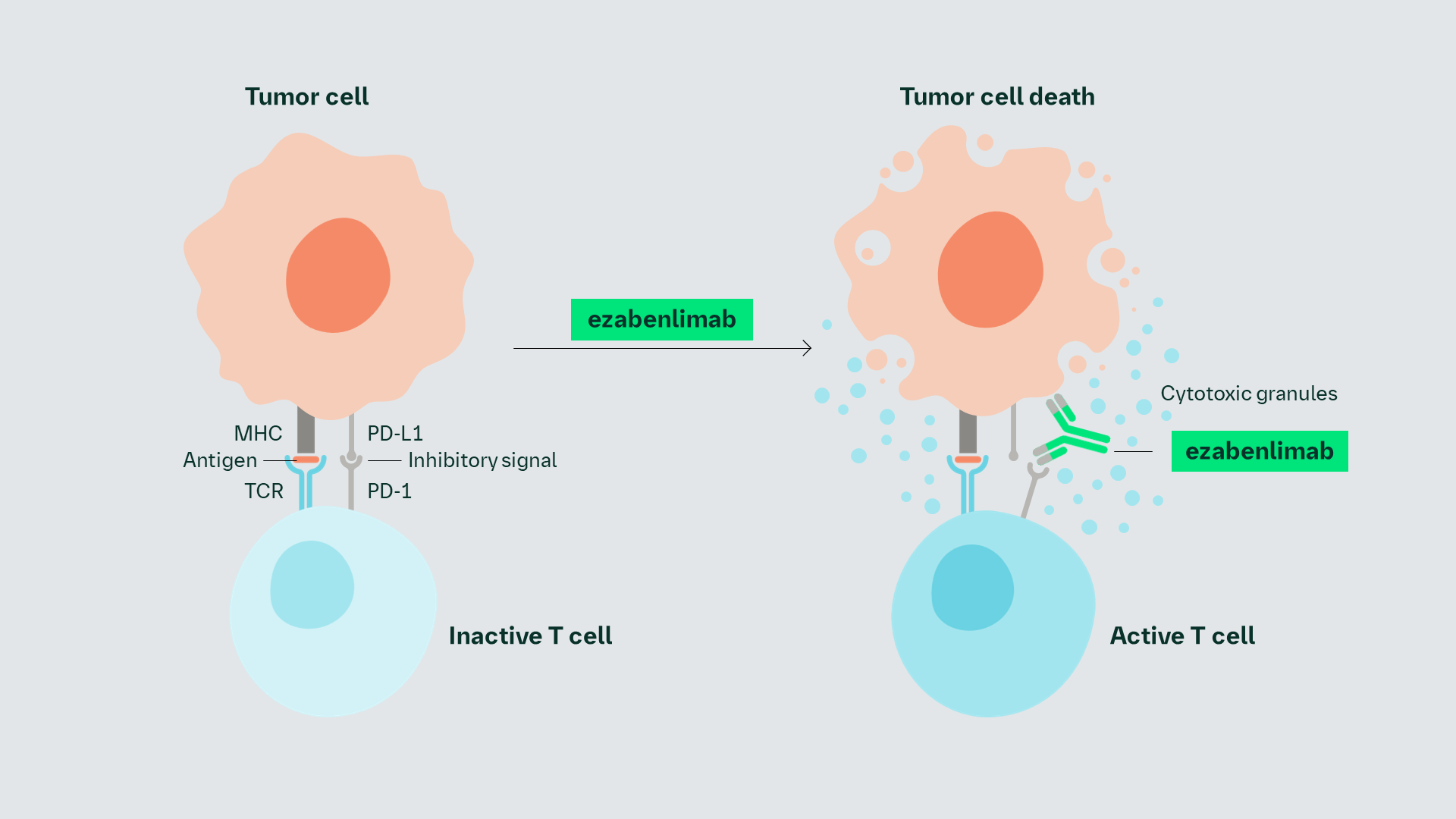
ezabenlimab is being investigated as a humanized PD-1-targeting monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2.1
Proposed MoA
T cells are inactivated by the interaction of PD-1 and PD-L1.1 ezabenlimab may block the interaction of PD-1/PD-L1, leading to activation of T cells.1 Activated T cells can secrete, for example, perforin and granzyme B to kill tumor cells.2
Combination therapy rationale
ezabenlimab is being investigated as monotherapy and as a backbone combination partner for multiple immuno-oncology approaches, including:
Monotherapy Proposed MoA1
1. Zettl M, et al. Cancer Res. 2018. 78(suppl 13):4558–4558; 2. Martinez-Lostao L, et al. Clin Cancer Res. 2015;21:5047–56.
Clinical Research and Development Trials (monotherapy and combinations): ezabenlimab is being investigated as a monotherapy for solid tumors in a Phase I trial.2 Phase I and II trials of ezabenlimab in combination with brigimadlin [BI 907828] (a murine double minute 2 [MDM2]-p53 antagonist),*3 BI 765049 (a B7-H6/CD3 T-cell engager),*4 BI 765179 (a CD137 FAP agonist),*5 signal-regulatory protein alpha [SIRPα] antagonists,*6-9 VSV-GP,*10 KISIMA™ cancer vaccine,*11 and BI 1703880 (a STING agonist)*12 are ongoing.
*This is an investigational compound and has not been approved. Its safety and efficacy have not been established.
ezabenlimab Combinational Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
1 | brigimadlin (MDM2-p53 antagonist) + ezabenlimab | Advanced solid tumors | Active, not recruiting | |
1/2 | DLL3/CD3 TcE (BI 764532) + ezabenlimab (PD-1 inhibitor) | Advanced SCLC and other NECs expressing DLL3 | Recruiting | |
1 | B7-H6/CD3 TcE (BI 765049) ± ezabenlimab | Advanced solid tumors expressing B7-H6 | Recruiting | |
1 | CD137 FAP agonist (BI 765179) ± ezabenlimab | Advanced solid tumors | Recruiting | |
1 | SIRPα antagonist (BI 765063) ± ezabenlimab | Advanced solid tumors that have a SIRPα polymorphism, including at least one V1 allele | Completed recruitment | |
1 | SIRPα antagonist (BI 765063) + ezabenlimab ± BI 836880, chemotherapy or cetuximab | Recurrent or metastatic HNSCC or HCC | Recruiting | |
1 | SIRPα antagonist (BI 765063 or BI 770371) + ezabenlimab | Advanced HNSCC, NSCLC, or melanoma | Recruiting | |
1 | SIRPα antagonist (BI 770371) ± ezabenlimab | Advanced solid tumors | Recruiting | |
1 | VSV-GP (BI 1831169) ± ezabenlimab | Solid tumors | Recruiting | |
1b | KISIMATM cancer vaccine (ATP150/ATP152/VSV-GP154) ± ezabenlimab | PDAC with KRAS G12D or KRAS G12V mutation | Recruiting | |
1 | STING agonist (BI 1703880) ± ezabenlimab | Advanced solid tumors | Recruiting |
ezabenlimab is being investigated as a humanized anti–PD-1 mAb that may block the interaction between PD-1 and its ligand PD-L1, leading to activation of T cells, which kill tumor cells1
In vivo, BI 754091 demonstrated dose-dependent tumor growth inhibition in MC-38 tumor-bearing mice. Safety was assessed in cynomolgus monkeys where repeated high doses of BI 754091 were well tolerated1
The MTD/RP2D of ezabenlimab was 240 mg q3w2
ezabenlimab showed clinical activity in patients with advanced solid tumors, with response rates that are consistent with other PD-1 inhibitors in similar populations2
ezabenlimab was well-tolerated, with a similar safety profile to other PD-1 inhibitors2
‒ Grade 3 TRAEs occurred in 6% of patients
ezabenlimab is currently undergoing clinical investigations in combination with other anti-cancer therapies, for the treatment of various solid cancers
1. Zettl M, et al. AACR 2018. Poster 4558; 2. Patel M, et al. ESMO 2021. Poster 542.
Zettl M, et al. Cancer Res. 2018. 78(suppl 13):4558–4558.
Martinez-Lostao L, et al. Clin Cancer Res. 2015;21:5047–5056.
Patel M, et al. ESMO 2021. Poster 542.
Johnson ML, et al. JSMO 2018. Oral Presentation.
Zettl M, et al. AACR 2018. Poster 4558.