KISIMA™ Cancer Vaccine
A modular, self-adjuvanting, protein-based cancer vaccine that has the potential to strengthen the capability of the patient’s immune system to recognize and kill tumor cells
KISIMATM Cancer Vaccine Proposed MoA
| Status | Phase 1 |
|---|---|
| Patient population | PDAC |
| Combination partners | PD-1 inhibitor-ezabenlimab |
Molecule
This compound is designed to be a modular, self-adjuvanting, peptide-based cancer vaccine that has the potential to strengthen the capability of the patient’s immune system to recognize and kill tumor cells.1–3 Designed using the KISIMATM technology platform, the investigational vaccine includes three components1–3 :
A cell-penetrating peptide for antigen delivery
A multi-antigenic cargo that is tailored to raise an immune response against colorectal tumors
A TLR peptide agonist as an adjuvant
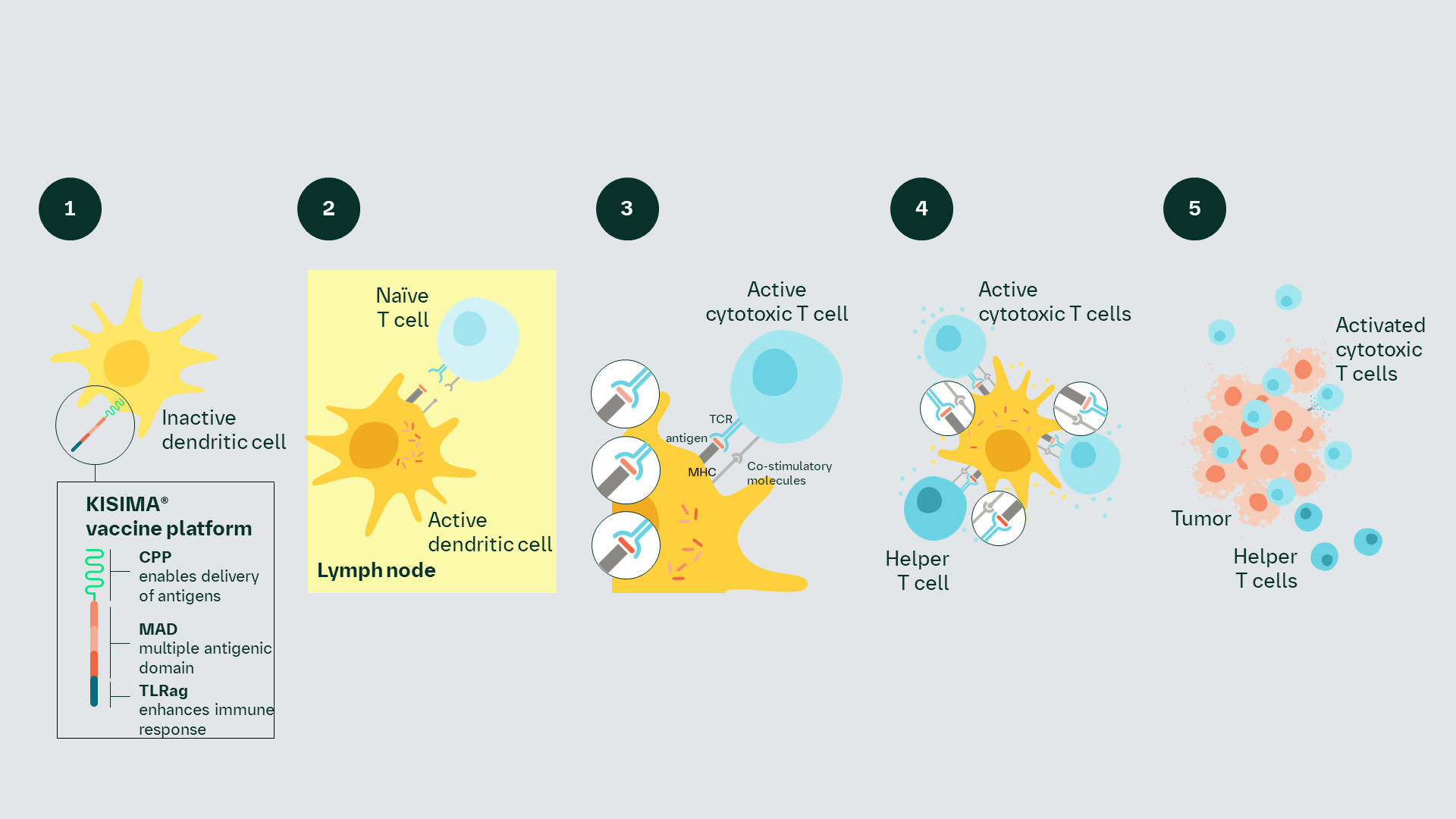
Proposed MoA
The investigational vaccine is engineered to induce an efficient immune response and promote immunological memory via activation of cytotoxic T cells and helper T cells.1–3
The cell penetrating peptide facilitates delivery of the investigational vaccine into dendritic cells. The TLR component may induce the upregulation of costimulatory molecules activating dendritic cells. Cancer-specific antigens are processed and resulting epitopes presented to T cells. Cytotoxic T cells become primed and educated to recognize and target tumor cells, and helper T cells become primed, triggering the release of cytokines. Cytokine release from T cells, as well as dendritic cells, leads to a cytotoxic T-cell response.
Proposed MoA1
Combination therapy rationale
Preclinical evidence supports the immunogenicity of a previous generation of the cancer vaccine in an in vivo model of CRC.4 This may lead to immunological memory and high vaccine efficacy, with increased intratumoral leukocyte infiltration.4
Combination with PD-1 blockade has been shown to have an additive effect, and may significantly increase the efficacy of the vaccine in vivo.1 As the vaccine enhances T-cell infiltration, this could sensitize tumors that are resistant to PD-1 inhibition (some of which have been shown to have limited immune infiltration) to checkpoint inhibitors.1,4
Note: this information reflects KISIMA-1. Patients are now being enrolled into KISIMA-2.
1. Boehringer Ingelheim. Data on file; 2. Boehringer Ingelheim. Press release. https://www.boehringer-ingelheim.com/press-release/acquisition-amal-therapeutics. Accessed October 2023; 3. AMAL Therapeutics. Therapeutic Vaccines. http://amaltherapeutics.com/science/therapeutic-vaccines/. Accessed October 2023; 4. Belnoue E, et al. JCI Insight. 2019;4:e127305.
Clinical Research and development
KISIMA™ Cancer Vaccine Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
NCT05846516 (KISIMA-02) | 1b | KISIMA™ cancer vaccine (ATP150/ATP152/VSV-GP154) ± ezabenlimab (PD-1 inhibitor) | PDAC with KRAS G12D or KRAS G12V mutation | Recruiting |
Boehringer Ingelheim. Data on file.
Boehringer Ingelheim. Press release. https://www.boehringer-ingelheim.com/press-release/acquisition-amal-therapeutics. Accessed October 2023.
AMAL Therapeutics. Therapeutic Vaccines. http://amaltherapeutics.com/science/therapeutic-vaccines/. Accessed October 2023.
Belnoue E, et al. JCI Insight. 2019;4:e127305.
You may also be interested in...

OUR PIPELINE