Results From Pooled Phase 1 Trials
Pooled Phase 1 Safety Data
The MTD/RP2D was ezabenlimab 240 mg Q3W
From a total of 111 patients who received treatment, 107 (96%) experienced AEs, the most common of which were fatigue (39%), nausea (29%), anemia (22%), and decreased appetite (20%)
64 patients (58%) had TRAEs, and grade 3 TRAEs were reported in 7 patients (6%) [decreased appetite; arthralgia; rash; AST increased; weight increased; allergic dermatitis and rash; stomatitis and diarrhea, (n=1 each)]
Serious AEs were reported in 38 patients (34%), 2 of which were treatment-related (grade 2 pyrexia and grade 3 rash)
Immune-related AEs were observed in 33 patients (30%); most commonly hypothyroidism (7 patients [6%]). Five patients (5%) had grade 3 immune-related AEs
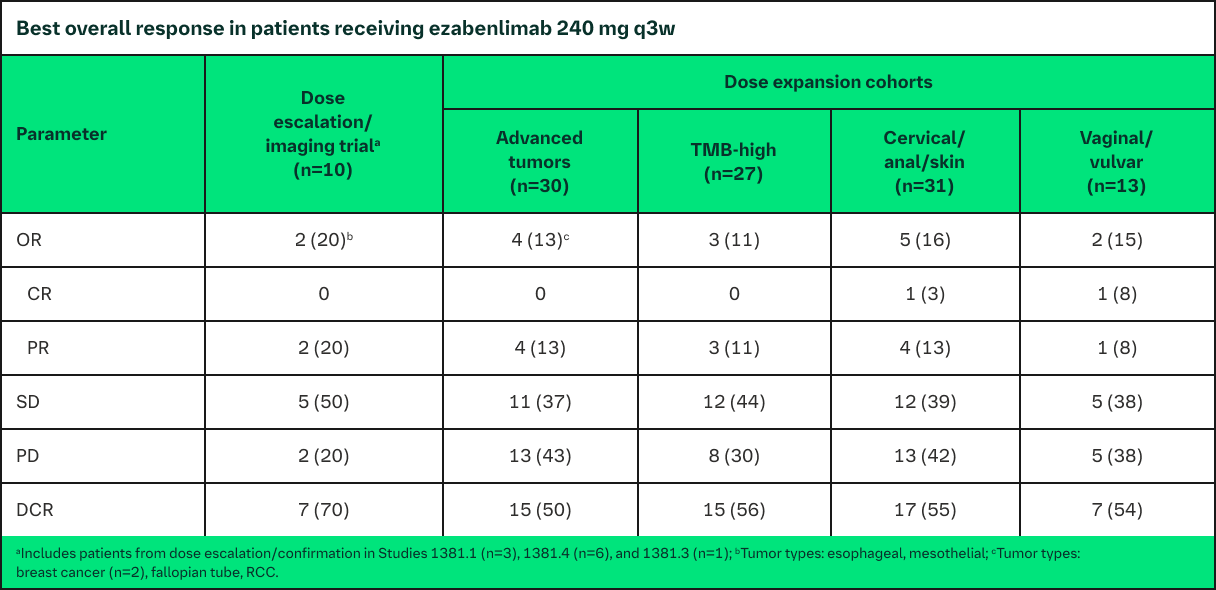
Pooled Phase 1 Efficacy Data
As of November 2020 cutoff, 98 (88%) patients discontinued treatment, most commonly due to PD (70%)
DOR ranged from 43 to 570 days
Patel M, et al. ESMO 2021. Poster 542.