DLL3/CD3 T-cell Engager
A bridge between DLL3-expressing tumor cells and cytolytic T cells
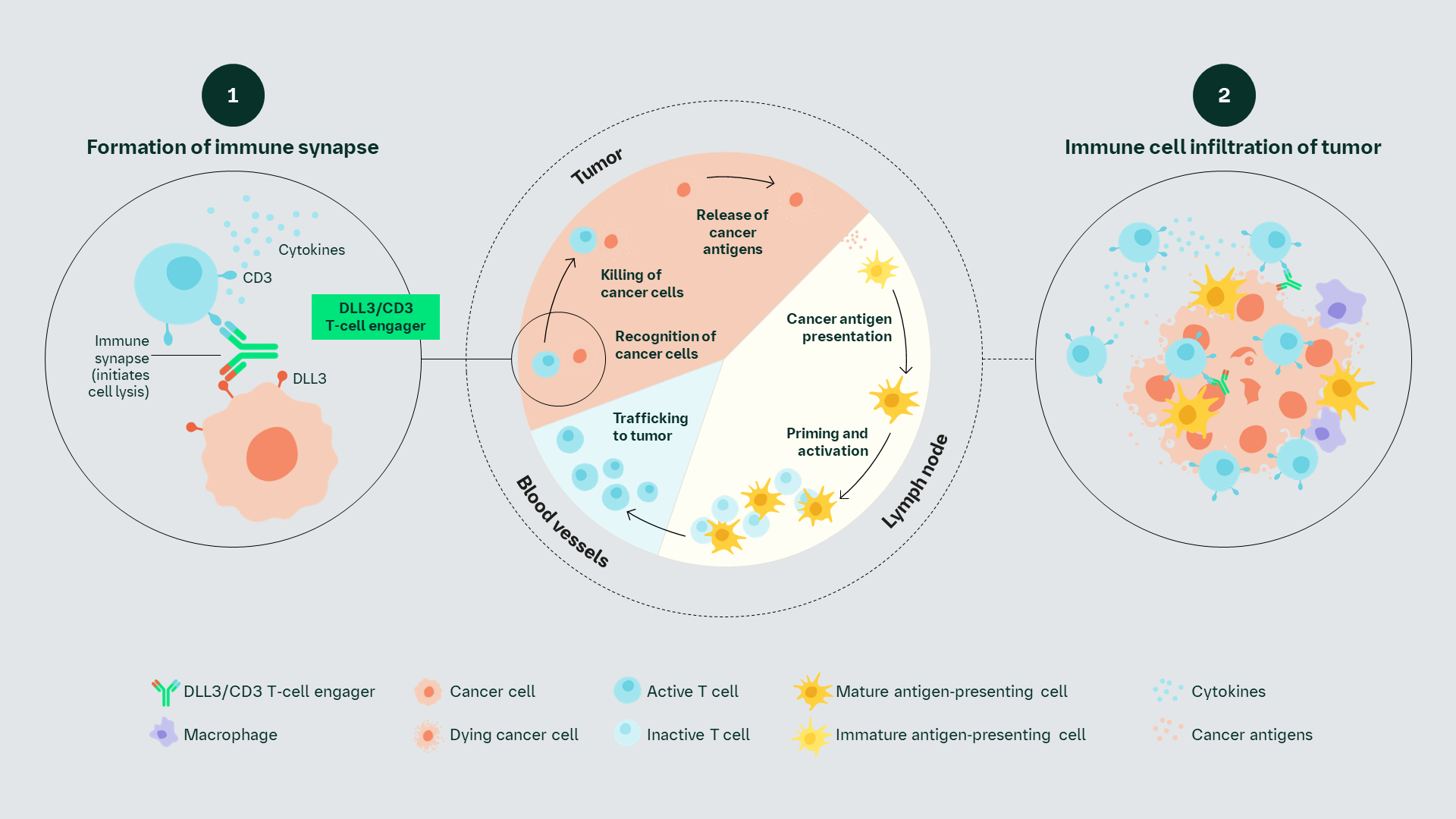
DLL3/CD3 T-cell Engager Proposed MoA
| Status | Phase 2 |
|---|---|
| Patient population | SCLC and other neuroendocrine carcinomas, glioma |
| Combination partners | ezabenlimab (PD-1 inhibitor) and other combinations |
Molecule
The DLL3/CD3 T-cell engager acts as a bridge between DLL3-expressing tumor cells and cytolytic T cells.1,2 This compound has an extended half-life and directs activity of cytolytic T cells selectively to DLL3-expressing tumors.1,2
Proposed MoA
The pharmacologic effect of the DLL3/CD3 T-cell engager depends on its ability to bind simultaneously to CD3 on T cells and to DLL3 expressed on tumor cells, resulting in the formation of a cytolytic synapse.1,2
DLL3 is an inhibitory Notch ligand that is expressed in tumors with a neuroendocrine origin, such as SCLC and glioblastoma multiforme, but not in normal adult tissue.3–5
Preclinical studies demonstrate the antitumor activity of the DLL3/CD3 T-cell engager in a range of DLL3-positive tumor models.2
Combination therapy rationale
The binding of the DLL3/CD3 T-cell engager to CD3 may lead to PD-1 upregulation in activated T cells and PD-L1 upregulation on malignant cells.2 Preclinical evidence supports the combination of the DLL3/CD3 T-cell engager with PD-1 inhibitors in order to revert this upregulation.1,2
Proposed MoA1,2
1. Ellerman D. Methods. 2019;154:102–117; 2. Hipp S, et al. Clin Cancer Res. 2020;26:5258–5268; 3. Hwang J, et al. J Clin Oncol. 2022;40(16_Suppl):4127; 4. Yao J, et al. Oncologist. 2022;27:940–951; 5. Xiu MX, et al. Oncotargets Ther. 2020;13:3881–3901.
DLL3/CD3 T-cell Engager Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
2 | BI 764532 monotherapy | Relapsed/refractory SCLC, and other relapsed/refractory NECs | Recruiting | |
1 | BI 764532 + chemotherapy in first time | Advanced NECs | Recruiting | |
NCT06077500 (DAREON™-8, 1438.8) | 1 | BI 764532 + cis/carboplatin + etoposide + atezolizumab/durvalumab | Extensive-stage SCLC | Recruiting |
NCT05990738 (DAREON™-9, 1438.9) | 1 | BI 764532 + SOC | Relapsed/refractory extensive-stage SCLC | Recruiting |
1 | BI 764532 monotherapy | Advanced SCLC and other NECs expressing DLL3 | Recruiting | |
1 | BI 764532 + ezabenlimab | SCLC and other neuroendocrine carcinomas that are positive for DLL3 | Recruiting | |
NCT05963876 (1438.4) | 1 | BI 764532 monotherapy | SCLC and other NECs | Recruiting |
NCT05916313 (1438.3) | 1b | BI 764532 monotherapy | Brain tumor, Glioma that is positive for DLL3 | Recruiting |
NCT05990738 (1438.9) | 1b | BI 764532 + topetecan | Relapsed/refractory extensive-stage SCLC | Recruiting |
DLL3/CD3 is a novel, half-life–extended, IgG-like T-cell engager that induces the formation of a cytolytic synapse by binding concomitantly to DLL3 on tumor cells and to CD3 on T cells, thereby selectively targeting DLL3-positive neoplasms1
In vivo, DLL3/CD3 monotherapy potently inhibited tumor growth in DLL3-positive SCLC xenograft models in a dose-dependent and time-dependent manner. It also modulated the inflammatory environment in the tumor tissue by redirecting CD4-positive and CD8-positive T-cell cytotoxicity toward DLL3-postitive SCLC cells, without affecting DLL3-negative target cells1
BI 764532 is being evaluated in an ongoing Phase 1 study in patients with DLL3-expressing SCLC and neuroendocrine carcinomas.2 As of August 14, 2023, 132 patients had been treated3
– The safety profile of BI 764532 is acceptable and manageable at clinically efficacious dose levels3,4
– Incidence of TRAEs was driven by CRS events, which were mostly Grade 1–2, occurred during initial drug administrations, and were manageable with standard supportive care3,4
– Promising efficacy was observed at doses ≥90 µg/kg3
– Responses appear to be durable3,4
– Further dose optimization is ongoing3,4
DAREONTM-5 is an ongoing Phase 2 dose-selection trial of BI 764532 in relapsed/refractory extensive-stage SCLC, LCNEC, and other relapsed/refractory NECs5
DAREONTM-8 is a planned Phase 1 dose-escalation study of BI 764532 with standard therapy in patients with extensive-stage SCLC, while DAREONTM-9 is a dose-escalation study of BI 764532 with topotecan in patients with relapsed/refractory extensive-stage SCLC6,7
1. Hipp S, et al. Clin Cancer Res. 2020;26:5258–5268; 2. NCT04429087. https://clinicaltrials.gov/ct2/show/NCT04429087. Accessed October 2023; 3. Gambardella V, et al. ESMO 2023. Oral Presentation 725MO; 4. Wermke M, et al. ASCO 2023. Oral Presentation 8502; 5. NCT05882058. https://clinicaltrials.gov/ct2/show/NCT05882058. Accessed October 2023; 6. NCT06077500. https://clinicaltrials.gov/ct2/show/NCT06077500. Accessed October 2023; 7. NCT05990738. https://clinicaltrials.gov/ct2/show/NCT05990738. Accessed October 2023.
Ellerman D. Methods. 2019;154:102–117.
Hipp S, et al. Clin Cancer Res. 2020;26:5258–5268.
Hwang J, et al. J Clin Oncol. 2022;40(16_Suppl):4127.
Yao J, et al. Oncologist. 2022;27:940–951.
Xiu MX, et al. Oncotargets Ther. 2020;13:3881–3901.
NCT04429087. https://clinicaltrials.gov/ct2/show/NCT04429087. Accessed April 2024.
NCT05882058. https://clinicaltrials.gov/ct2/show/NCT05882058. Accessed April 2024.
NCT05990738. https://clinicaltrials.gov/ct2/show/NCT05990738. Accessed April 2024.
NCT05879978. https://www.clinicaltrials.gov/study/NCT05879978. Accessed April 2024.
NCT06132113. https://clinicaltrials.gov/study/NCT06132113. Accessed April 2024.
NCT05916313. https://clinicaltrials.gov/study/NCT05916313. Accessed April 2024.
NCT06077500. https://clinicaltrials.gov/study/NCT06077500. Accessed April 2024.
Van den Bent M, et al. Society for NeuroOncology Annual Meeting 2023. Poster CTIM-30.
Capdevila J, et al. AACR 2024. Poster CT090.
Wermke M, et al. ASCO 2021. Poster TPS8588.
Hipp S, et al. AACR 2019. Poster 549.
Mazières J, et al. ESMO 2023. Poster 2028TiP.
Gambardella V, et al. ESMO 2023. Oral Presentation 725MO.
Gambardella V, et al. NANETS 2023. Oral Presentation 23713.
Wermke M, et al. WCLC 2023. Oral Presentation OA01.05.
Wermke M, et al. ASCO 2023. Oral Presentation 8502.
Peters S, et al. ASCO 2024. Poster TPS8127.
Boehringer Ingelheim. Data on file.
