HER2 TKI-zongertinib
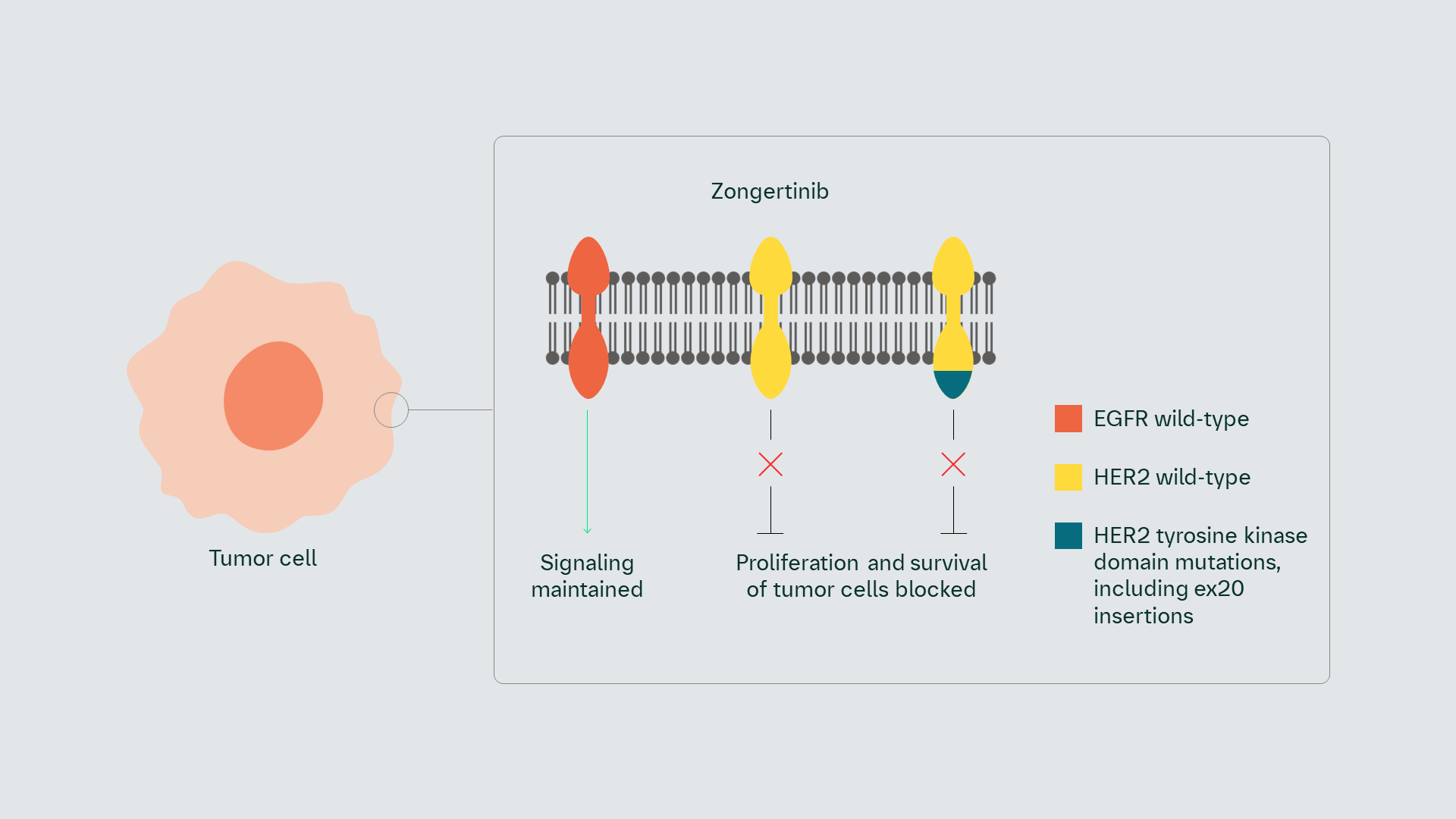
Zongertinib (BI 1810631) is a novel, orally administered human epidermal growth factor receptor 2 (HER2) tyrosine kinase inhibitor (TKI) that selectively and covalently binds to the tyrosine kinase domain (TKD) of both wild-type and mutated HER2 receptors, while sparing wild-type EGFR.
HER2 TKI-zongertinib Proposed MoA
| Status | Phase I-Phase III |
|---|---|
| Patient population | NSCLC, Solid Tumors |
Molecule
Zongertinib (BI 1810631) is a novel, orally administered HER2 TKI that selectively and covalently binds to the TKD of both wild-type and mutated HER2 receptors, including those with an exon 20 insertion, while sparing wild-type EGFR.
Proposed MoA
Mutations of ErbB receptor tyrosine kinases, such as EGFR and HER2, can cause aberrant signaling in cancer cells through uncontrolled proliferation, inhibition of apoptosis, and promotion of tumor growth and spread.3–6
Zongertinib (BI 1810631) may selectively and covalently bind to the tyrosine kinase domain of mutated HER2 receptors.1 Selective binding has the potential to block aberrant downstream signaling, while sparing wt EGFR signaling, thereby avoiding wt EGFR-associated toxicity.1
Pan-ErbB TKIs are impeded by wt EGFR-associated toxicity. Improved selectivity of HER2 TKI compared to second generation TKIs may result in better tolerability and potential for more effective dosing.1
Clinical Development
Zongertinib (BI 1810631) is currently undergoing clinical investigation as a monotherapy in patients with HER2 aberrations for advanced or metastatic solid tumors.1,2,7
Proposed MoA1
A Phase III, open-label, randomized, active-controlled, multicenter trial (Beamion LUNG-2) is evaluating orally administered zongertinib compared with SOC as first-line treatment in patients with unresectable, locally advanced or metastatic nonsquamous NSCLC harboring HER2 TKD mutations.7
Beamion LUNG-1 is a Phase I trial evaluating zongertinib in the treatment of advanced or metastatic solid tumors with HER2 alterations.
Beamion BCGC-1: A Phase Ib Dose Escalation and Phase II Dose Optimization, Randomized, Open-label, Multicenter Trial of Oral Zongertinib (BI 1810631) in Combination With Intravenous Trastuzumab Deruxtecan (T-DXd) or in Combination With Intravenous Trastuzumab Emtansine (T-DM1) for Treatment of Patients With Advanced HER2+ Metastatic Breast Cancer (mBC) and Metastatic Gastric, Gastroesophageal Junction, or Esophageal Adenocarcinoma (mGEAC).
Zongertinib Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
1 | zongertinib [BI 1810631] monotherapy | Advanced or metastatic HER2 aberrations in solid tumors | Recruiting | |
3 | zongertinib [BI 1810631] monotherapy | Advanced or metastatic nonsquamous NSCLC harboring HER2 TKD alterations | Recruiting | |
1/2 | zongertinib + trastuzumab deruxtecan (T-DXd) or trastuzumab emtansine (T-DM1) | HER2 overexpressing and/or amplified (HER2+), metastatic breast cancer (mBC) or metastatic gastric adenocarcinoma, | Recruiting |
Zongertinib (BI 1810631) selectively and covalently binds to the TKD of mutated HER2 receptors. Selective binding blocks aberrant downstream signaling, while sparing wt EGFR signaling, thereby avoiding wt EGFR-associated toxicity1
Zongertinib (BI 1810631) is being evaluated in the Phase I Beamion LUNG-1 study in patients with advanced or metastatic NSCLC with HER2 aberrations1,2
- As of January 29, 2024, 83 patients have been treated in Phase 1a, while 42 patients have been treated in Phase Ib Cohort as of July 31, 20231
- In the Phase Ia escalation part, MTD was not reached with either BID or QD schedule1
- Doses taken into dose optimization are 240 mg and 120 mg QD1
- Zongertinib (BI 1810631) demonstrated encouraging preliminary antitumor activity in various cancers with HER2 aberrations, including NSCLC1
Beamion LUNG-2 is a Phase III, open-label, randomized, active-controlled, multicenter trial evaluating orally administered zongertinib compared with SOC as first-line treatment in patients with unresectable, locally advanced or metastatic nonsquamous NSCLC harboring HER2 TKD mutations3,4
1. Heymach J, et al. NACLC 2023. OA01.28; 2. NCT04886804. https://clinicaltrials.gov/ct2/show/study/NCT04886804. Accessed April 2024; 3. NCT06151574. https://clinicaltrials.gov/study/NCT06151574. Accessed April 2024; 4. Wu Y, et al. AACR 2024. Poster CT284.
Opdam F, et al. AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Symposium 2022. #1LBA.
Heymach J, et al. Clin Lung Cancer. 2023;24:e65–e68.
Wilding B, et al. Nature Cancer. 2022;3:821–836.
Tai W, et al. J Control Release. 2010;146:264–275.
Gazdar AF. Cancer Metastasis Rev. 2010;29:37–48.
Connell CM, Doherty GJ. ESMO Open. 2017;2:e000279.
NCT04886804. Available at https://clinicaltrials.gov/ct2/show/study/NCT04886804. Accessed April 2024.
NCT06151574. Available at https://clinicaltrials.gov/study/NCT06151574. Accessed April 2024.
Ruiter G, et al. ESMO 2023. Poster 1375P.
Yamamoto N, et al. WCLC 2023. Abstract MA13.08.
Heymach J, et al. ASCO 2023. Poster 8545.
Heymach J, et al. AACR 2023. Poster CT203.
Heymach J, et al. AACR 2022. Poster CT212.
Heymach J, et al. NACLC 2023. OA01.28.
Neumüller RA, et al. AACR 2021. Poster 1472.
Wu Y, et al. ELCC 2024. Poster 34P.
Wu Y, et al. AACR 2024. Poster CT284.
Johnson M, et al. ASCO 2024. Poster TPS8654.
Heymach J, et al. ASCO 2024. Rapid Oral Abstract 8514.
You may also be interested in…

OUR PIPELINE

NSCLC IN FOCUS
