Phase I dose-escalation study of brigimadlin (MDM2-p53 antagonist) + ezabenlimab in patients with advanced solid tumors (NCT03964233)
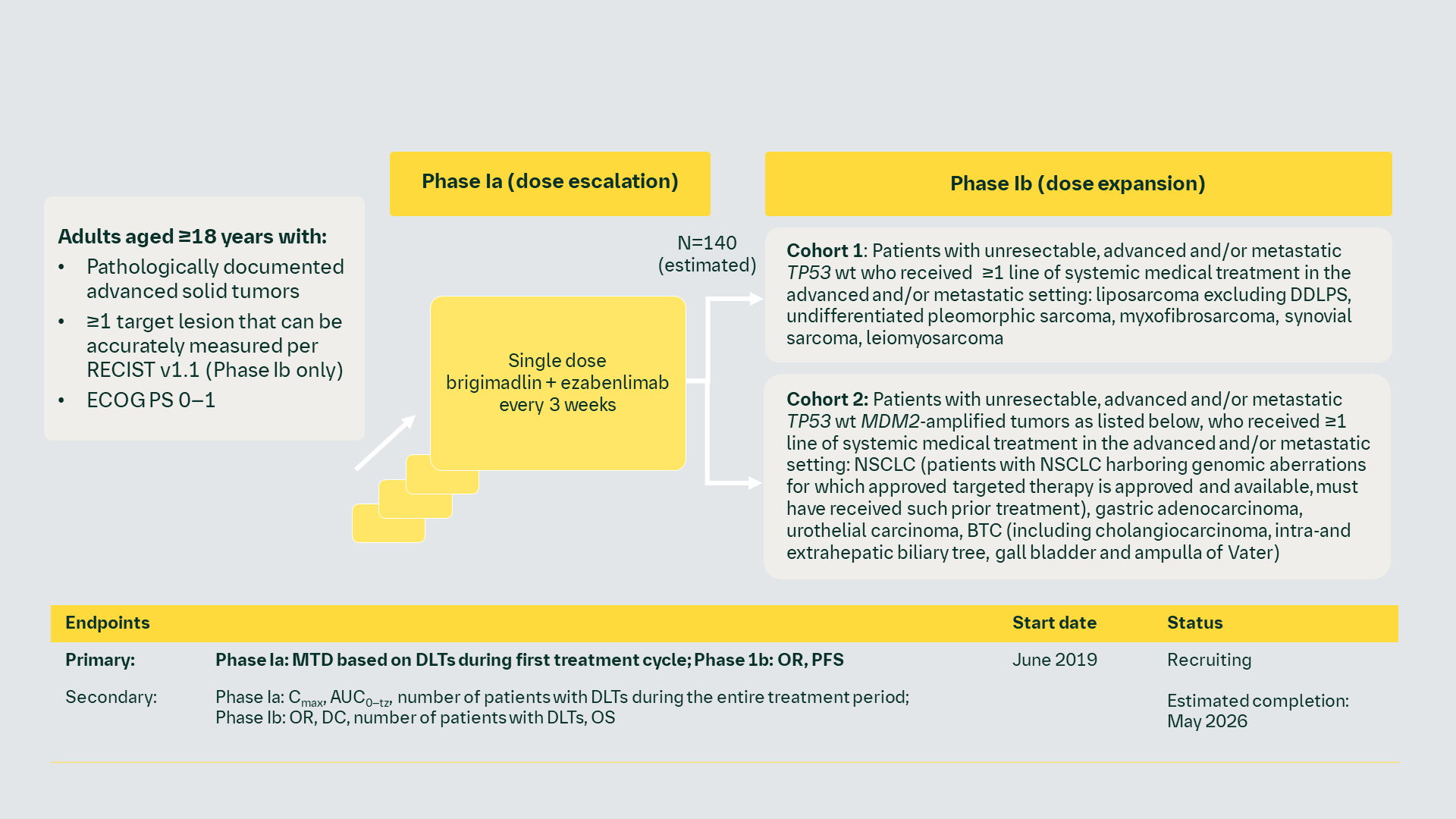
DC, disease control; DLT, dose-limiting toxicity; ECOG PS, Eastern Cooperative Oncology Group performance status; MDM2, murine double minute 2; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; OR, objective response; PFS, progression-free survival; RECIST, Response Evaluation Criteria In Solid Tumors; wt, wild type.