MDM2-p53 Antagonist-brigimadlin
An orally administered MDM2-p53 antagonist that binds to MDM2 and may block the MDM-p53 interaction, thus possibly preventing MDM2 from inactivating p53 and thereby, restoring p53 function1,2
MDM2-p53 Antagonist-brigimadlin Proposed MoA
| Status | Phase III: DDLPS |
|---|---|
| Patient population | Glioblastoma (GBM), Lung adenocarcinoma, DDLPS |
| Combination partners | PD-1 inhibitor-ezabenlimab |
Molecule
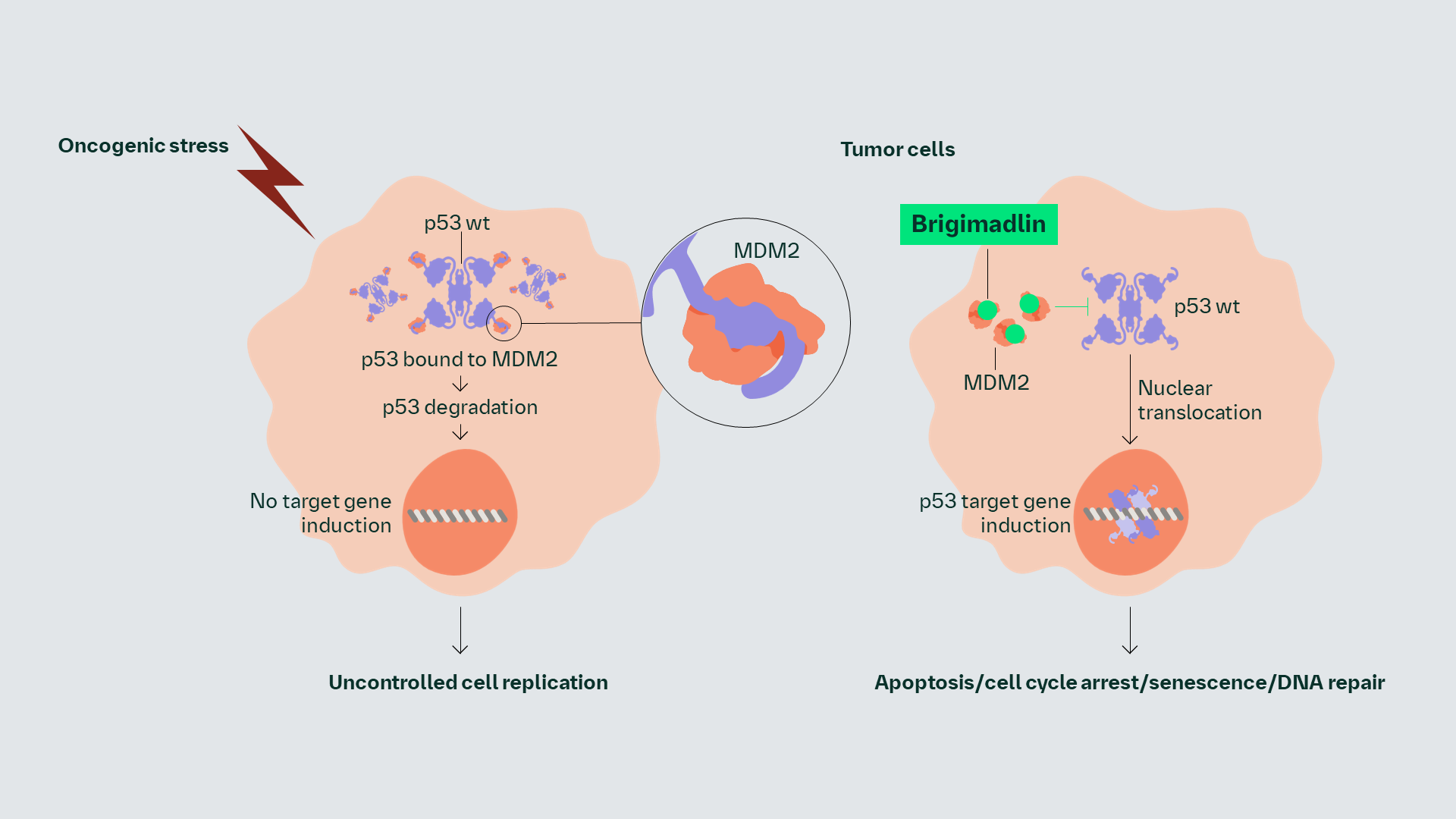
Brigimadlin is an orally administered MDM2-p53 antagonist.
Brigimadlin binds to MDM2 and may block the MDM2–p53 interaction; this may prevent MDM2 from inactivating p53, thereby restoring p53 function.1,2
Proposed MoA
The MDM2 oncoprotein is a critical regulator of the tumor suppressor p53.3 Overexpression of MDM2 promotes p53 degradation and aids tumor proliferation.2,3
Brigimadlin may block the interaction between MDM2 and p53. This may prevent MDM2 from inactivating p53, thereby restoring p53 function.1 Stabilization of p53 leads to TP53 target gene induction that subsequently leads to cell cycle arrest or apoptosis in tumor cells with TP53 wild type status.1,4,5
Preclinical and Clinical Development
Preclinical study results have shown that brigimadlin had single-agent efficacy in MDM2-amplified DDLPS PDX models and improved responses/disease control in patient-derived xenografts and syngeneic mouse tumor models.5–7
Proposed MoA3–5,8–10
Dose escalation and expansion stuides in Phase I clinical trials have shown encouraging preliminary antitumor activity and a management safety profile.7,11
Brigimadlin is being investigated in a Phase II study (Brightline-2), and a Phase III study (Brightline-4), and a Phase 1 study in glioblastoma.
1. Gounder M, et al. CTOS 2022. Oral Presentation #19; 2. Schöffski P, et al. Future Oncol. 2023;19:621–629; 3. Zhao Y, et al. Acta Biochim Biophys Sin (Shanghai). 2014;46:180–189; 4. Schöffski P, et al. ASCO 2022. Poster TPS11586; 5. Rudolf D, et al. AACR 2018. Poster 4866; 6. Cornillie J, et al. Clin Transl Oncol. 2020;22:546–554; 7. Gounder M, et al. ASCO 2022. Abstract 3004; 8. Marei HE, et al. Cancer Cell Int. 2021;21:703; 9. LoRusso P, et al. ASCO 2021. Poster 3016; 10. Wang HQ, et al. Cancer Res. 2008;78(suppl 13): Abstract 5560; 11. Goyal L, et al. ASCO 2023. Poster TPS4179; 12. NCT05218499. https://clinicaltrials.gov/ct2/show/NCT05218499. Accessed April 2024; 13. NCT05512377. https://clinicaltrials.gov/ct2/show/NCT05512377. Accessed April 2024; 14. NCT06058793. https://classic.clinicaltrials.gov/ct2/show/NCT06058793. Accessed April 2024.
Brigimadlin (MDM2-p53 Antagonist) Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
2/3 | brigimadlin vs doxorubicin | Advanced dedifferentiated liposarcoma | Completed Recruitment | |
2 | brigimadlin monotherapy | Biliary tract adenocarcinoma and other solid tumors (pancreatic ductal carcinoma, lung adenocarcinoma, bladder cancer) | Recruiting* *Currently recruiting for Lung adenocarcinoma exploratory cohort | |
3 | brigimadlin monotherapy | Advanced dedifferentiated liposarcoma | On hold | |
0/1a | brigimadlin monotherapy | Newly diagnosed glioblastoma | Recruiting | |
1 | brigimadlin monotherapy | Advanced solid tumors | Active, not recruiting | |
1 | brigimadlin + ezabenlimab (PD-1 inhibitor) | Advanced solid tumors | Active, not recruiting |
Brigimadlin is currently being investigated as an MDM2-p53 antagonist that targets tumors by promoting p53-mediated cell cycle arrest
and apoptosis1,2Preclinical studies showed that brigimadlin had single-agent efficacy in MDM2-amplified DDLPS PDX models3
Based on preliminary data from the Phase 1 trials, brigimadlin demonstrated a manageable safety profile and preliminary antitumor activity4,5
– The MTD was 60 mg and the RDE was selected as 45 mg given once every 3 weeks4
– In patients with MDM2-amplified DDLPS, preliminary median PFS was 7.9 months, ORR was 14.5%, and DCR was 82.6%5
The efficacy and safety of MDM2-p53 antagonist in DDLPS is being investigated in lung adenocarcinoma (Cohort 3), Brightline-2 study
The efficacy and safety of brigimadlin in patients with advanced BTC are also being evaluated in the Phase 2 Brightline-2 trial after preliminary tumor responses were observed in earlier studies6
A Phase 0/1a dose-escalation study of brigimadlin in combination with radiotherapy is currently recruiting patients with newly diagnosed glioblastoma7,8
Brightline-4 is a Phase 3 study of brigimadlin in patients with advanced DDLPS that is anticipated to start in December 20239
1. Gounder M, et al. CTOS 2022. Oral Presentation #19; 2. Schöffski P, et al. Future Oncol. 2023;19:621–629; 3. Cornillie J, et al. Clin Transl Oncol. 2020;22:546–554; 4. Gounder M, et al. ASCO 2022. Abstract 3004; 5. LoRusso P, et al. ASCO 2023. Poster 11554; 6. Goyal L, et al. ASCO 2023. Poster TPS4179. 7. Sarkaria J, et al. ASCO 2023. Poster TPS2081; 8. NCT05376800. https://clinicaltrials.gov/ct2/show/NCT05376800 Accessed October 2023; 9. NCT06058793. https://clinicaltrials.gov/ct2/show/NCT06058793. Accessed October 2023.
Gounder M, et al. CTOS 2022. Oral Presentation #19.
Schöffski P, et al. Future Oncol. 2023;19:621–629.
Zhao Y, et al. Acta Biochim Biophys Sin (Shanghai). 2014;46:180–189.
Schöffski P, et al. ASCO 2022. Poster TPS11586.
Cornillie J, et al. Clin Transl Oncol. 2020;22:546–554.
Gounder M, et al. ASCO 2022. Abstract 3004.
Marei HE, et al. Cancer Cell Int. 2021;21:703.
LoRusso P, et al. ASCO 2021. Poster 3016.
Wang HQ, et al. Cancer Res. 2018;78(suppl 13): Abstract 5560.
Schöffski P, et al. ESMO Sarcoma and Rare Cancers 2023. Oral Presentation 42O.
Tolcher A, et al. CTOS 2022. Poster 167.
Santoro M, et al. AACR 2024. Poster CT288.
NCT03964233. https://clinicaltrials.gov/ct2/show/NCT03964233. Accessed April 2024.
NCT03449381. https://clinicaltrials.gov/ct2/show/NCT03449381. Accessed April 2024.
NCT05512377. https://clinicaltrials.gov/ct2/show/NCT05512377. Accessed April 2024.
NCT05218499. https://clinicaltrials.gov/ct2/show/NCT05218499. Accessed April 2024.
NCT05376800. https://clinicaltrials.gov/ct2/show/NCT05376800. Accessed April 2024.
NCT06058793. https://clinicaltrials.gov/ct2/show/NCT06058793. Accessed April 2024.
Boehringer Ingelheim. Data on file.
You may also be interested in ...

OUR PIPELINE

CONGRESS HUB
