SIRPα Antagonist
Humanized IgG monoclonal antibodies
SIRPα Antagonist Proposed MoA
| Status | Phase 1 |
|---|---|
| Patient population | Solid tumors |
| Combination partners | PD-1 inhibitor-ezabenlimab |
Molecule
The BI SIRPα program includes 2 mAbs that target the SIRPα receptor on the surface of myeloid cells, blocking the interaction between SIRPα and CD47.1 CD47 is an ubiquitous cell-surface protein that is highly expressed on some cancer cells, acting as a ‘don’t eat me’ signal.2,3
BI 765063 is an IgG4Pro mAb that may recognize only the V1 variant of SIRPα, while BI 770371 is an IgG1 mAb that may recognize both the V1 and V2 variants of SIRPα.1
Proposed MoA
SIRPα is a regulatory protein expressed on the surface of macrophages and other myeloid immune cells.2 The interaction between SIRPα and CD47 serves as a myeloid-specific immune checkpoint, inhibiting macrophage activation and migration of myeloid cells such as monocytes, PMNs, and DCs.4,5
Disruption of the SIRPα/CD47 axis restores the immune functions of myeloid cells in the TME, increasing the phagocytosis of cancer cells. Enhanced phagocytosis also increases antigen uptake and presentation, linking the innate and adaptive immune systems.2,5
Proposed MoA1,5,6
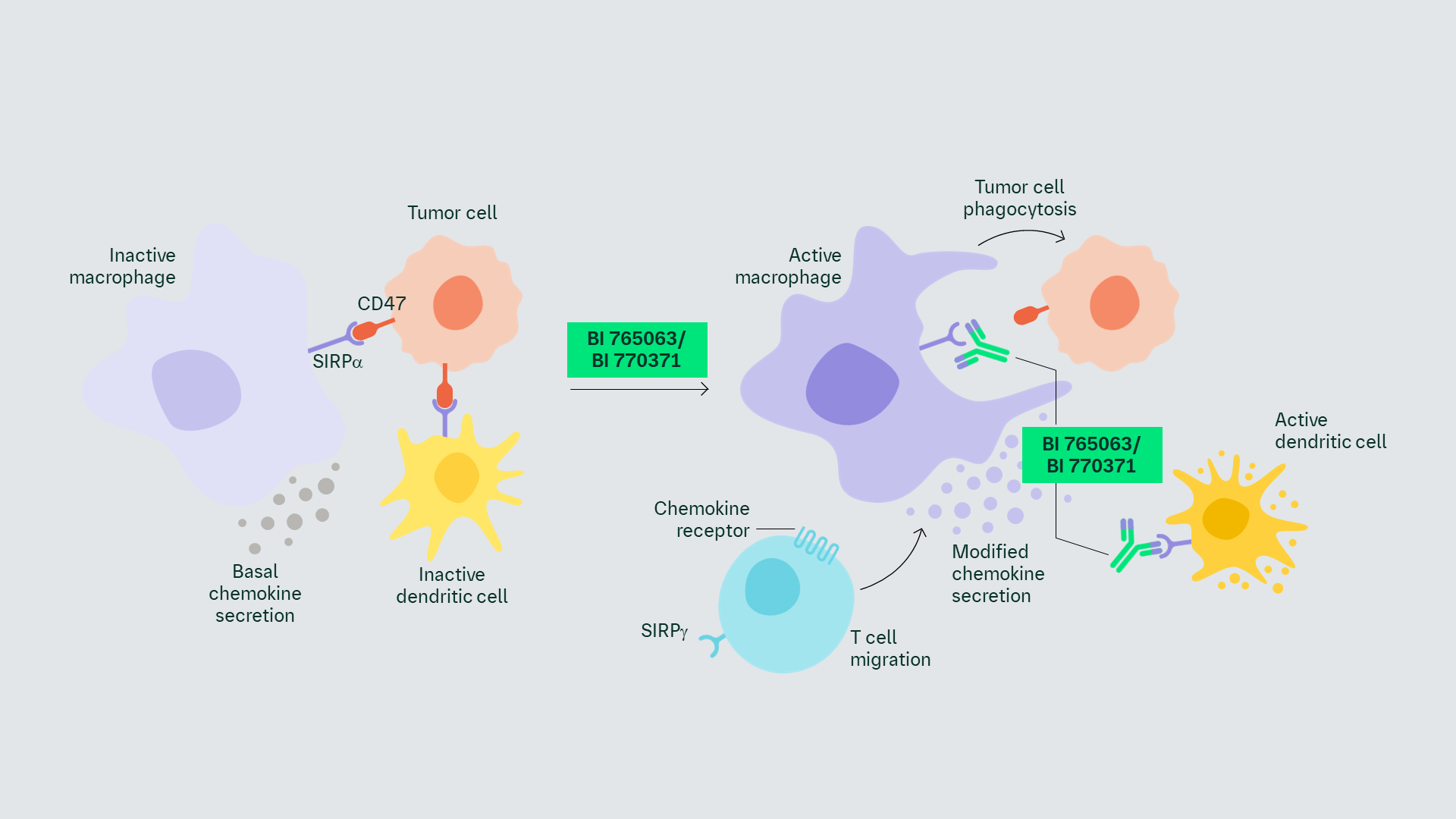
This link between innate and adaptive immunity points to combination therapy with an adaptive immune checkpoint inhibitor.2
Combination therapy rationale
Preclinical studies in mouse tumor models have demonstrated clinical benefit of SIRPα inhibitor monotherapy.5,6 These studies also indicate that clinical outcomes may be enhanced through combination therapy with a PD-1 inhibitor or with a costimulatory agent, such as an anti-4-1BB mAb, which would provide dual activation of innate and acquired immunity.5,6
1. Boehringer Ingelheim. Data on file; 2. Barclay AN, et al. Nat Rev Immunol. 2006;6:457–464; 3. Brown EJ, Frazier WA. Trends Cell Biol. 2001;11:130–135; 4. Majety M, et al. FEBS J. 2018;285:763–776; 5. Weiskopf K. Eur J Cancer. 2017;76:100–109; 6. Gauttier V, et al. Cancer Res. 2018;78(Suppl.): Abstract 1684.
Clinical Research and Development
SIRPα Antagonist Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
1 | BI 765063 ± ezabenlimab (PD-1 inhibitor) | Advanced solid tumors that have a SIRPα polymorphism, including at least one V1 allele | Completed recruitment | |
1 | BI 765063 ± ezabenlimab (PD-1 inhibitor) alone or with BI 836880, chemotherapy, or cetuximab | Recurrent or metastatic HNSCC or HCC | Completed recruitment | |
1 | BI 765063 or BI 770371 in combination with ezabenlimab (PD-1 inhibitor) | Advanced HNSCC, NSCLC, or melanoma | Recruiting | |
1 | BI 770371 ± ezabenlimab (PD-1 inhibitor) | Advanced solid tumors | Recruiting |
The BI SIRPα program includes 2 mAbs that target the SIRPα receptor on the surface of myeloid cells, intended to block the interaction between SIRPα and CD47.1 CD47 is an ubiquitous cell-surface protein that is highly expressed on some cancer cells, acting as a “don’t eat me” signal2,3
BI 765063 is an IgG4Pro mAb that is intended to recognize only the V1 variant of SIRPα, while BI 770371 is an IgG1 mAb that is intended to recognize both the V1 and V2 variants of SIRPα1
Combination with anti-SIRPα and anti–PD-L1 may result in increased number of T cells at the tumor periphery and increased infiltration of T cells to the core of the tumor in preclinical studies4
BI 765063 in combination with ezabenlimab (PD-1 inhibitor) showed preliminary anti-tumor activity in the Phase 1 study in patients with advanced solid tumors5,6
– BI 765063 was well-tolerated with no reported DLTs up to the highest dose tested5
– The RP2D of BI 765063 was determined as 24 mg/kg Q3W with full RO saturation6
BI 770371 is currently being investigated in a Phase 1 dose-escalation/expansion trial alone or in combination with ezabenlimab (PD-1 inhibitor) in patients with advanced solid tumors7
– The combination has been shown to be generally well-tolerated, with no DLTs observed during the MTD evaluation period
– MTD has not been reached and the trial is ongoing
1. Boehringer Ingelheim. Data on file; 2. Barclay AN, et al. Nat Rev Immunol 2006;6:457–64; 3. Brown EJ, Frazier WA. Trends Cell Biol. 2001;11:130–135; 4. Gauttier V et al. J Clin Invest. 2020;130:6109–6123; 5. Champiat S, et al. ASCO 2021. Poster 2623; 6. Kotecki N et al. ESMO 2021; Abstract 983P; 7. Gutierrez ME, et al. ESMO 2023. Poster 697P.
Barclay AN, et al. Nat Rev Immunol. 2006;6:457–64.
Brown EJ and Frazier WA. Trends Cell Biol. 2001;11:130–135.
Majety M, et al. FEBS J. 2018;285:763–776.
Weiskopf K. Eur J Cancer. 2017;76:100–109.
Gauttier V, et al. Cancer Res. 2018;78(suppl): abstract 1684.
Gauttier V et al. J Clin Invest. 2020;130:6109-6123.
Gutierrez ME, et al. ESMO 2023. Poster 697P.
Champiat S, et al. AACR 2023. Poster 2129.
Champiat S, et al. AACR 2022. Poster 1993.
Kotecki N, et al. ESMO 2021. Poster 983.
Champiat S, et al. ASCO 2021. Poster 2623.
NCT03990233. https://clinicaltrials.gov/ct2/show/NCT03990233. Accessed September 2023.
NCT05249426. https://clinicaltrials.gov/ct2/show/NCT05249426. Accessed September 2023.
NCT05068102. https://clinicaltrials.gov/ct2/show/NCT05068102. Accessed September 2023.
NCT05327946. https://clinicaltrials.gov/ct2/show/NCT05327946. Accessed September 2023.
Boehringer Ingelheim. Data on file.
You may also be interested in...

OUR PIPELINE