VSV-GP oncolytic virus
Targeting cancer with highly tumor-specific oncolytic viruses
VSV-GP oncolytic virus
Our VSV-GP oncolytic virus platform is an engineered oncolytic virus built upon VSV with a replaced surface glycoprotein, which acts by infecting and killing malignant cells and sparing their normal counterparts.1-4
VSV-GPP Oncolytic Virus Proposed MoA
| Status | Phase 1 |
|---|---|
| Patient population | Solid tumors |
| Combination partners | ezabenlimab (PD-1 inhibitor) |
Molecule
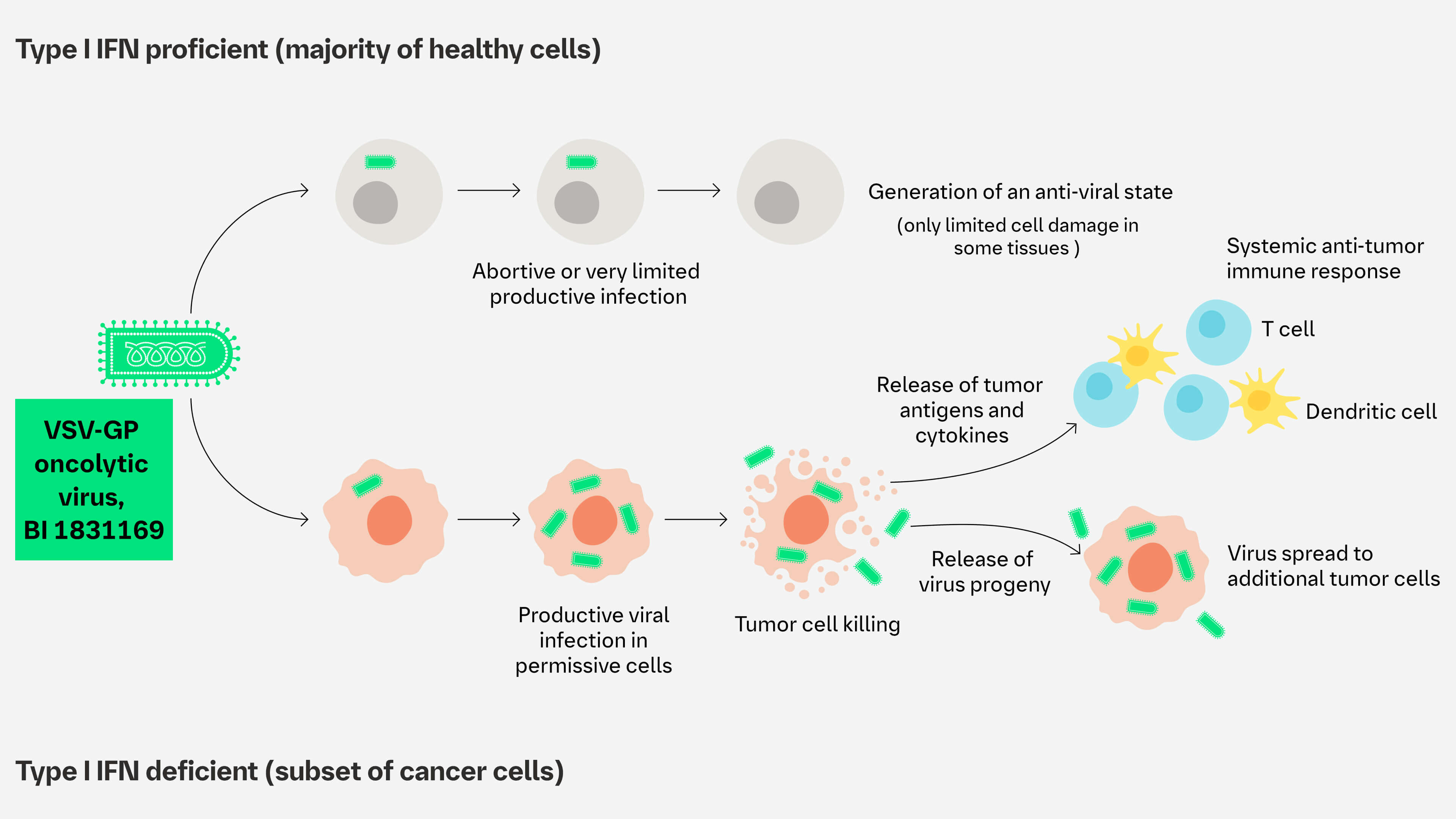
The VSV-GP oncolytic virus platform is an engineered oncolytic virus built upon a chimeric VSV with its surface protein replaced by the GP of the lymphocytic choriomeningitis virus. This engineered oncolytic virus is designed to mitigate the risk of neurotoxicity and neurotoxicity and lower the risk of developing neutralizing antibodies, allowing repeated intra-tumoral and systemic administration1,2
Proposed MoA
The VSV-GP is intended to induce immunogenic cell death in tumors susceptible to type I interferon and stimulate immune cell recruitment into tumors (turning cold tumors into hot tumors).1,2 Tumor cell–specific antigens are released via oncolysis, which, together with innate immune activation, induces anti-tumor activity. Furthermore, the VSV-GP does not integrate into the human genome.
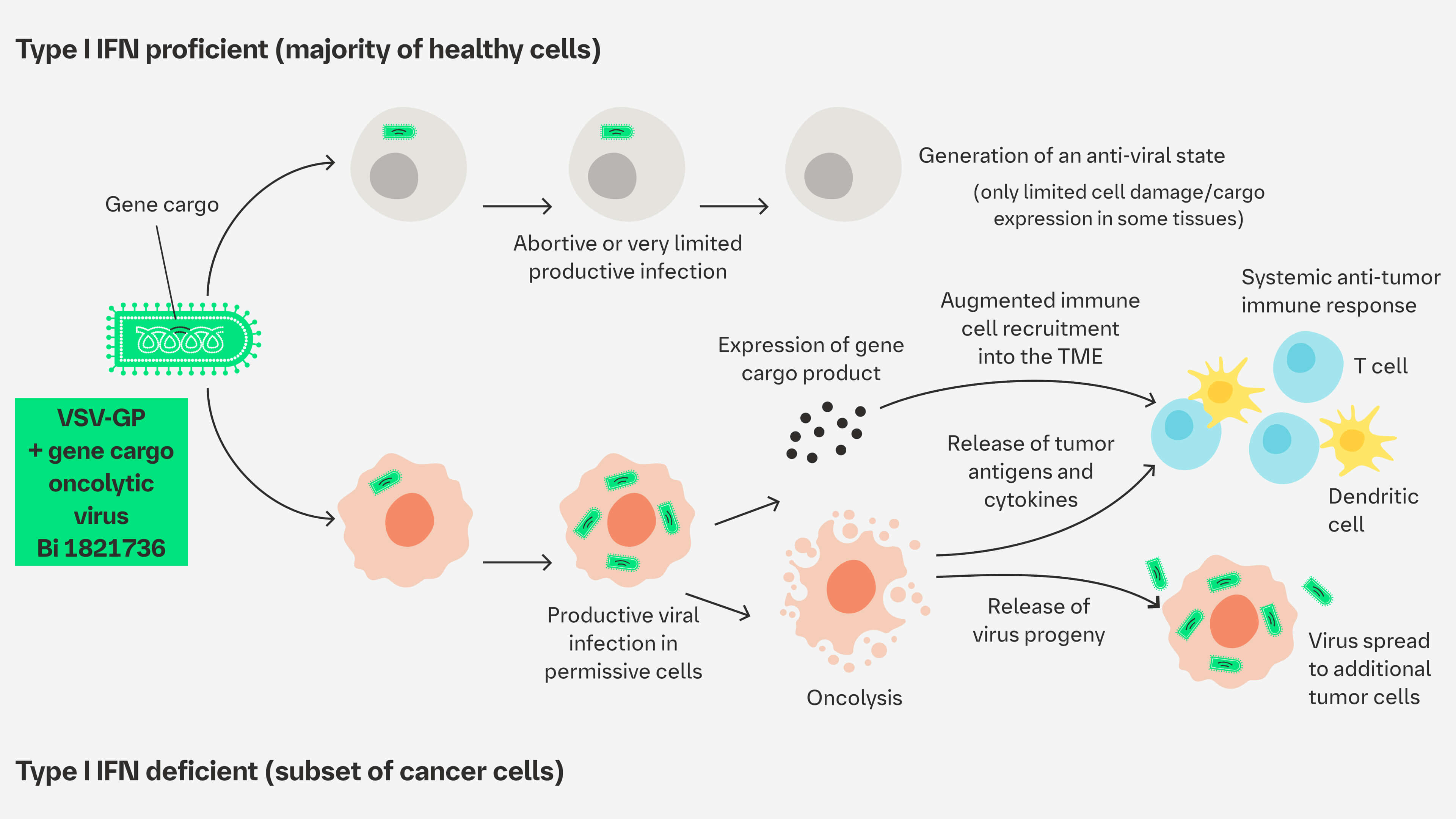
The viral platform can be armed with additional therapeutic genes (gene cargos). The local expression of gene cargos at the tumor site may enhance VSV-GP–mediated antitumor immune response.
Gene cargos can be introduced into the viral genome.2 The local expression of gene cargos at the tumor site may enhance VSV-GP–mediated anti-tumor immune response.4
Combination rationale
The VSV-GP has shown preclinical activity in a wide variety of solid cancer types, as well as synergy when combined with PD-1 blockade.1–4
Proposed MoA1,2
Proposed MoA1,2
1. Muik A, et al. Cancer Res. 2014;74:3567–3578; 2. Tober R, et al. J Virol. 2014;88:4897–4907; 3. Schreiber LM, et al. Br J Cancer. 2019;121:647–658; 4. Müller P, et al. Cancer Res 2020;80(16:suppl)4450.
Clinical Research and Development
VSV-GP Clinical Trials
| Trial number | Phase | Compound | Patient population | Status |
|---|---|---|---|---|
1 | BI 1831169 (VSV-GP) ± ezabenlimab (PD-1 inhibitor) | Solid tumors | Recruiting | |
1 | BI 1821736 (VSV-GP-CD80Fc) | Solid tumors | Recruiting |
VSV-GP may induce a pro-inflammatory microenvironment within infected tumors and increases T-cell infiltration1
VSV-GP may lead to tumor remission in an LLC1 lung cancer tumor model2
VSV-GP and VSV-GP with immune-stimulatory cargo are currently being evaluated in 2 Phase 1 clinical trials for patients with advanced solid tumors3,4
1. Müller P, et al. Cancer Res 2020;80(16 suppl):4450; 2. Schreiber LM, et al. Br J Cancer. 2019;121:647–658; 3. NCT05839600. https://clinicaltrials.gov/ct2/show/NCT05839600. Accessed October 2023; 4. Boehringer Ingelheim. Data on file.
Schreiber LM, et al. Br J Cancer. 2019;121:647–658.
Müller P, et al. AACR 2020. Poster 4450.
NCT05155332. https://clinicaltrials.gov/ct2/show/NCT05155332. Accessed April 2024.
NCT05839600. https://clinicaltrials.gov/ct2/show/NCT05839600. Accessed April 2024.
Tolcher A, et al. ASCO 2024. Poster TPS2688.
Boehringer Ingelheim. Data on file.
You may also be interested in…

OUR PIPELINE