Results of Phase 1443-0001 Monotherapy Trial
SIRPα antagonist (BI 765063) in patients with advanced solid tumors
Phase I safety data
BI 765063 was well-tolerated with no reported DLTs up to the highest dose tested. MTD was not reached
- No treatment-related thrombocytopenia and only one case
of anemia (grade 2) was observed1
- Most treatment-related IRRs were low grade and reversible1,2
The most frequent TRAEs were IRR (28%), fatigue (28%), arthralgia, maculo-papular rash (22%), and pruritus (17%)1
All treatment-related AEs were mild to moderate, except for one case of grade 3 maculopapular rash1
BI 765063 showed dose-proportional systemic exposure and full RO saturation in Cycle 1 at the 18 and 24 mg/kg dose levels1
(1443-0002) is being conducted with BI 765063 (SIRP-alpha) 1st GEN. All future studies will be with BI 770371 (SIRP-alpha) 2nd GEN.
Phase I efficacy data
BI 765063 showed preliminary antitumor activity in the Phase I study in patients with advanced solid tumors
- A combination of PR and SD responses were observed
in 21 of 47 (45%) evaluable patients (data as of June 2021)2
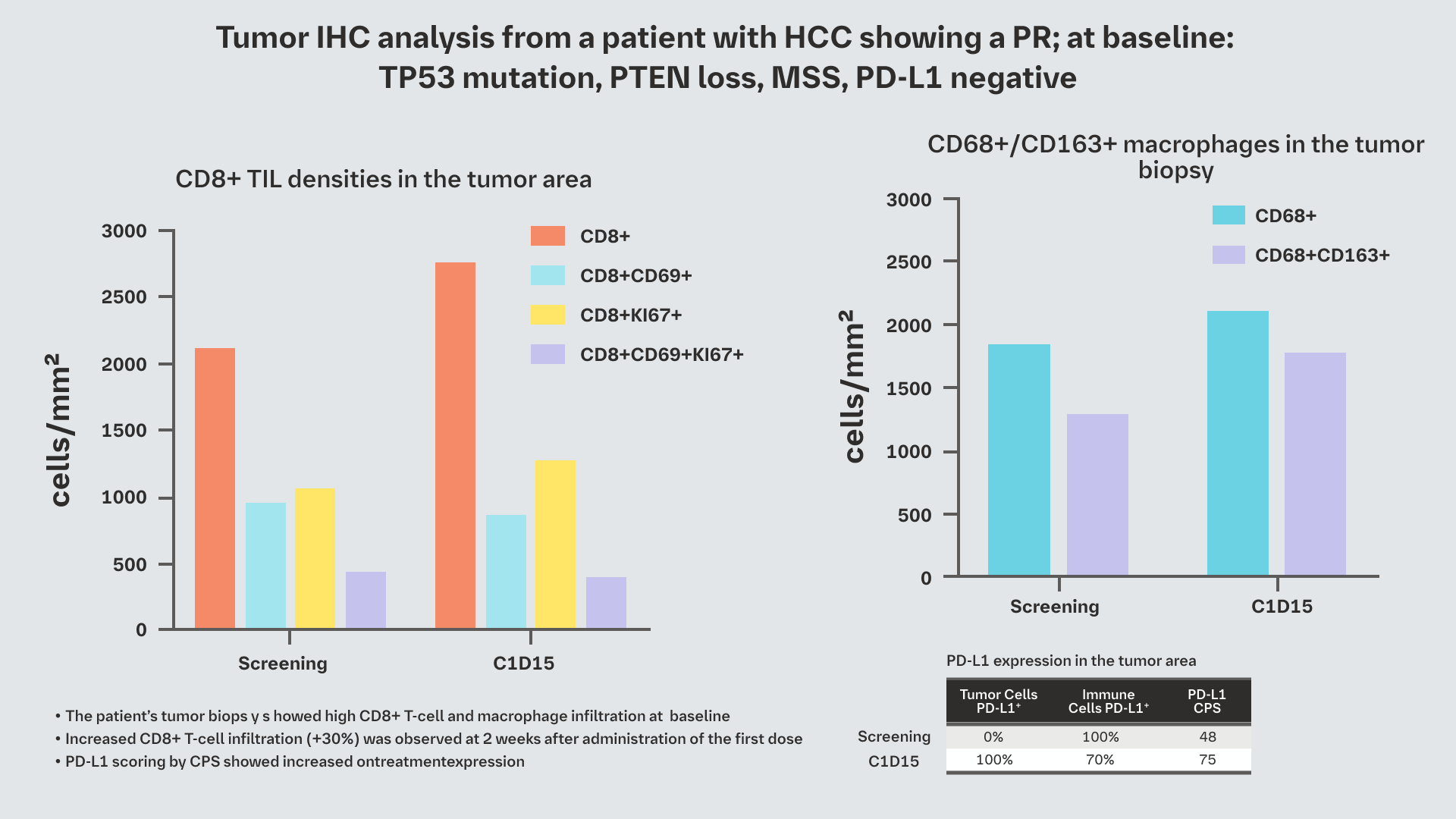
- One patient with HCC with liver and lung metastases and
seven prior lines of therapy showed a durable PR
maintained for >18 months
(treatment is still ongoing as of April 2022)3
Analysis of paired tumor biopsies in other patients is ongoing
1. Kotecki N, et al. ESMO 2021. Poster 983; 2. Champiat S, et al. ASCO 2021. Poster 2623; 3. Champiat S, et al. AACR 2022. Poster 1993.
Results of Phase 1443-0002 Combination Trial
SIRPα antagonist (BI 765063) in combination with ezabenlimab (PD-1 inhibitor) in patients with advanced solid tumors
Phase I safety data
BI 765063 in combination with the PD-1 inhibitor ezabenlimab was well tolerated with no DLTs. MTD was not reached
- No treatment-related thrombocytopenia
and only one case of anemia (grade 2) were observed
All BI 765063 TRAEs were grade 1 or 2, except grade 3 rash maculopapular in 1 patient; no grade 4/5 TRAEs were reported
The RP2D of BI 765063 was determined as 24 mg/kg Q3W with full RO saturation
Phase I efficacy data
BI 765063 in combination with PD-1 inhibitor ezabenlimab showed preliminary antitumor activity in the Phase 1 study in patients with advanced solid tumors1
- Two patients with endometrial cancer experienced
confirmed PRs and an additional patient with CRC had an iPR
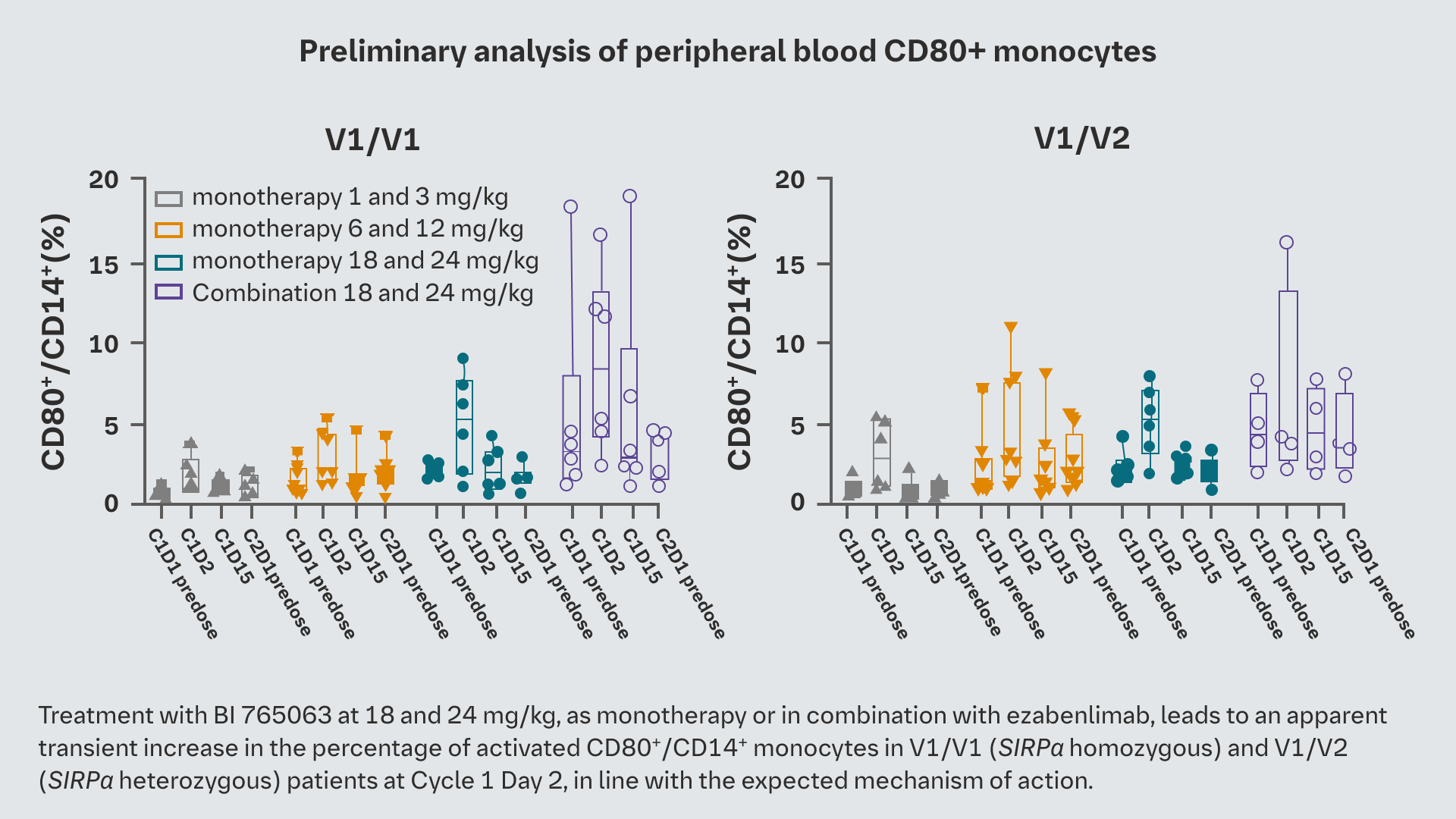
Treatment with BI 765063 at 18 and 24 mg/kg, as monotherapy or in combination with ezabenlimab, leads to an apparent transient increase in the percentage of activated CD80+/CD14+ monocytes in V1/V1 (SIRPα homozygous) and V1/V2 (SIRPα heterozygous) patients at Cycle 1 Day 2, in line with the expected MoA
Kotecki N, et al. ESMO 2021. Abstract 983P.