Clinical Results
1438.1: Preliminary data from the FIH trial
As of August 14, 2023, 132 patients had been treated1
- 54 patients (41%) have epNEC
- 30% of all patients had ≥3 prior lines of therapy
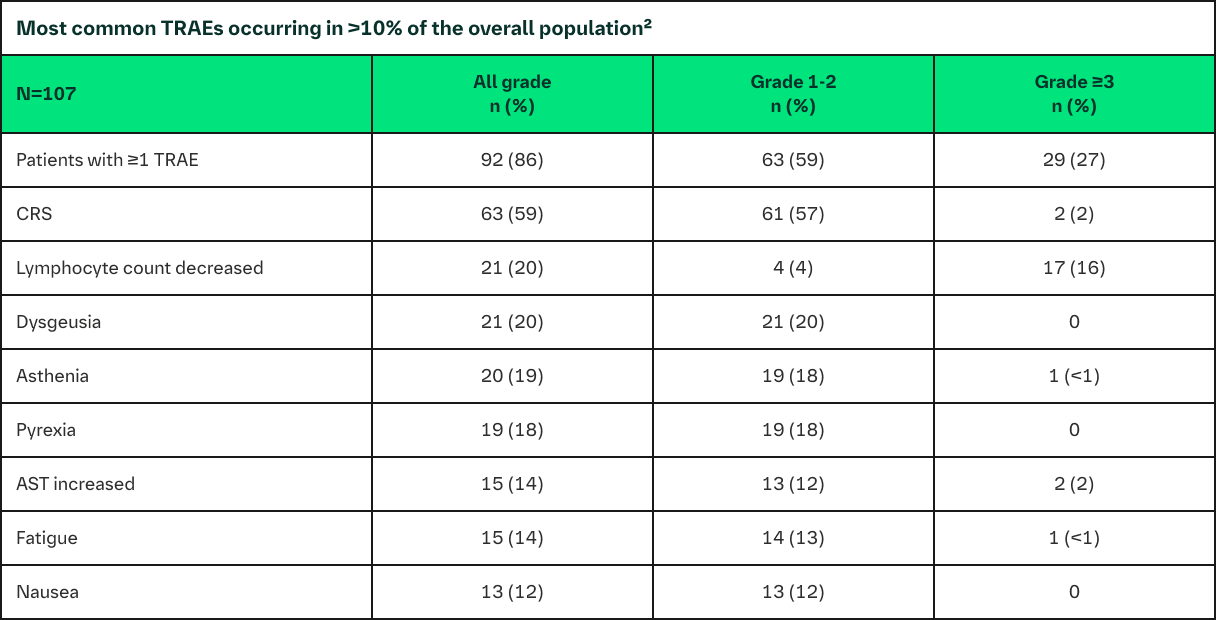
Safety
The safety profile of BI 764532 is manageable at clinically efficacious dose levels
Incidence of TRAEs was driven by CRS events1,2
– CRS cases were mostly Grades 1–2, which occurred during initial drug administrations
- All cases were manageable with standard supportive care
- DLTs were reversible and patients recovered1,2
- MTD has not been reached; dose escalation is ongoing1
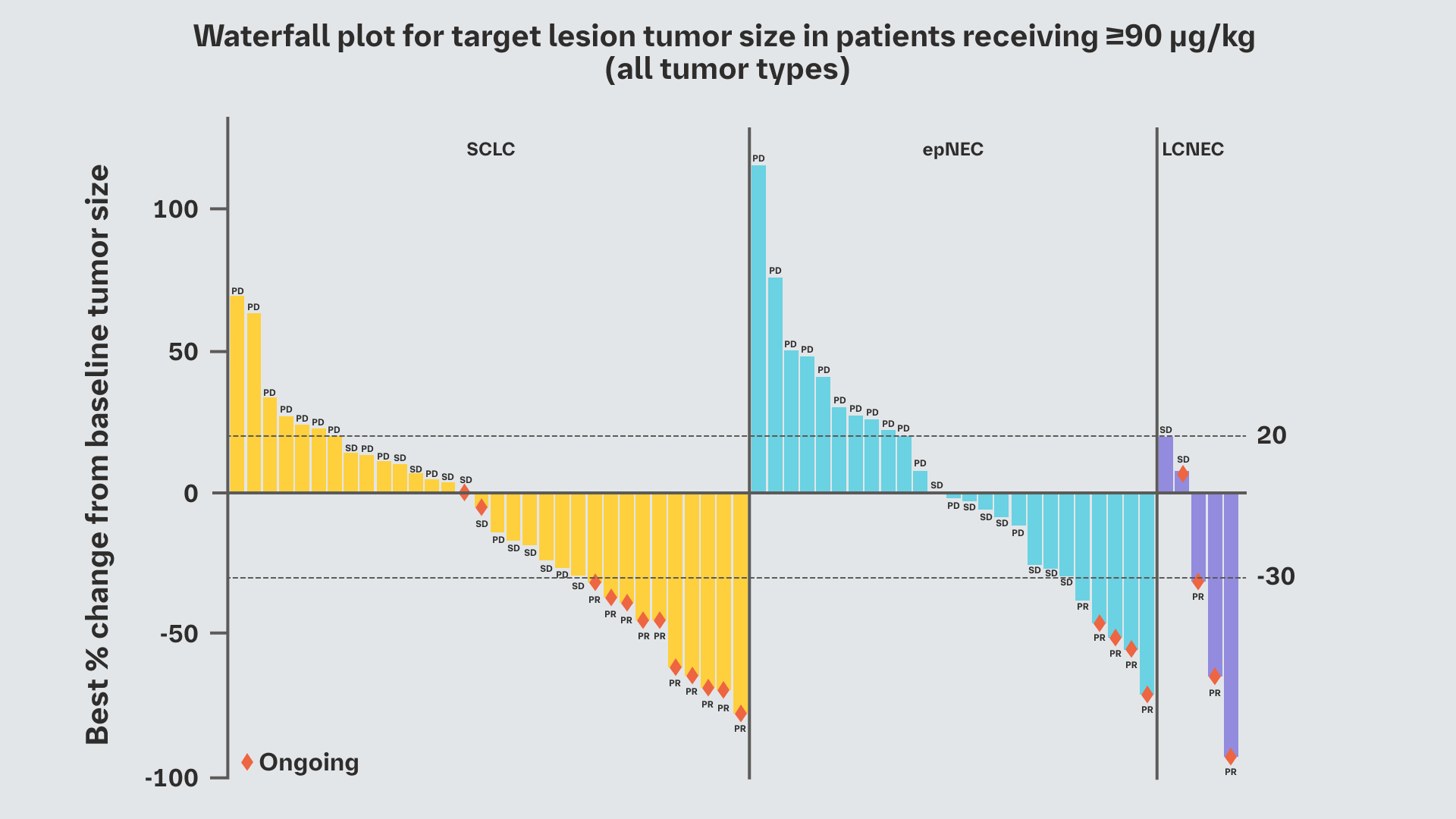
Efficacy
- Responses were seen in all tumor types enrolled2
Promising efficacy was observed at doses ≥90 μg/kg1,2
1. Gambardella V, et al. ESMO 2023. Oral Presentation 725MO; 2. Wermke M, et al. ASCO 2023. Oral Presentation 8502.
Preclinical Results
DLL3/CD3 T-cell engager in SCLC models
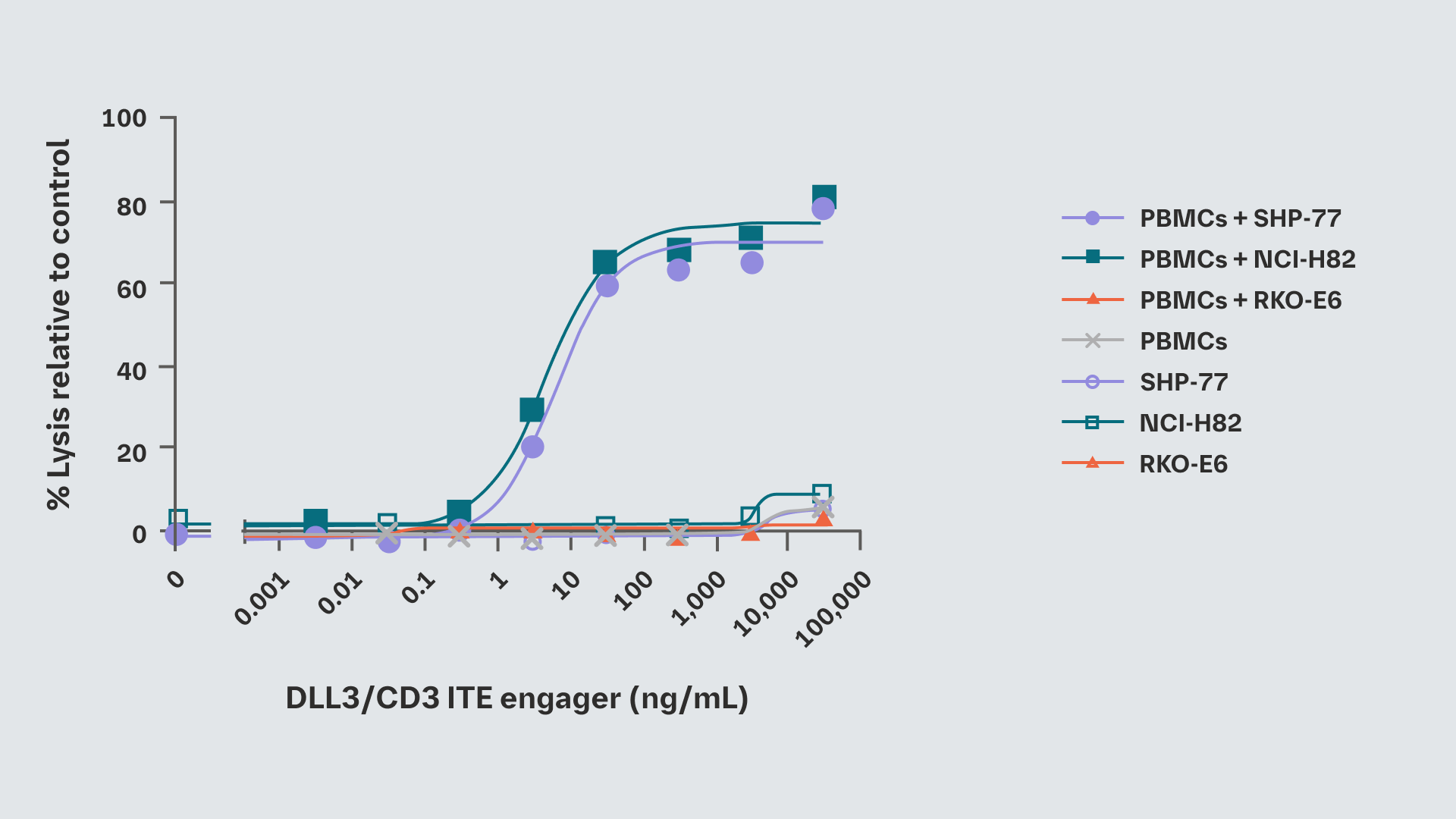
The DLL3/CD3 T-cell engager selectivity induced lysis of DLL3-positive SCLC cell lines SHP-77 and NCI-H82. In contrast, the viability of DLL3-negative cell line RKO-E6 was not affected
In vitro efficacy of DLL3/CD3 T-cell engager in SCLC cell lines
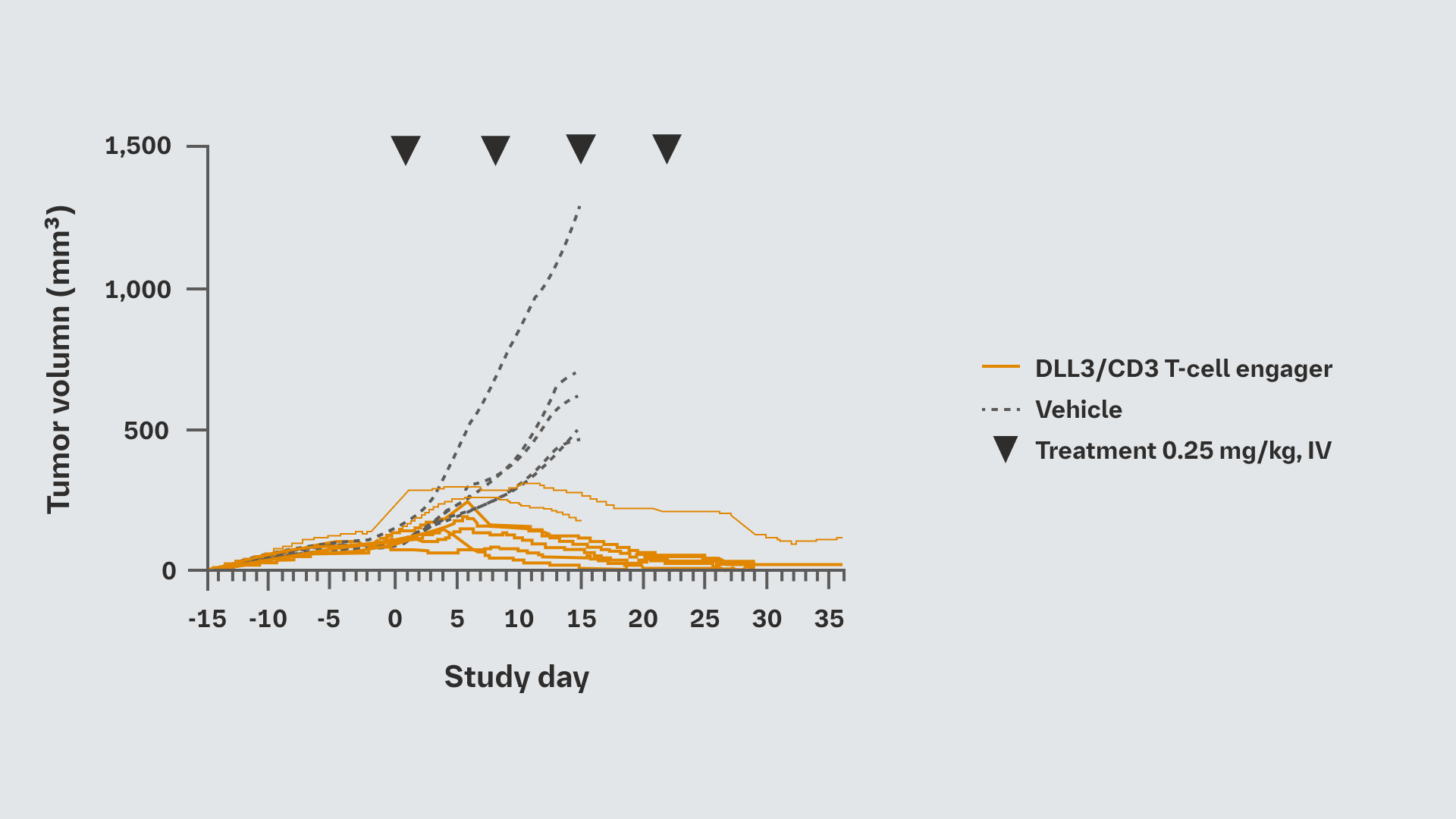
In vivo efficacy studies were conducted in a human SHP-77 xenograft model where the DLL3/CD3 T-cell engager was administered Q1W, as supported by its 20-day half-life in C57/BL6 mice
DLL3/CD3 monotherapy demonstrated dose-dependent anti-tumor activity, with statistically significant tumor growth inhibition compared with vehicle (P<0.05 at Day 15)
It also induced infiltration of T cells and upregulation of PD-(L)1 into tumor tissue, resulting in apoptosis of tumor cells
In vivo efficacy of DLL3/CD3 T-cell engager in humanized SCLC xenograft model
Hipp S, et al. Clin Cancer Res. 2020;26:5258–5268.