DAREON™-5 (1438.5): A Phase 2, randomized, open-label, dose-selection study of a DLL3/CD3 T-cell engager (BI 764532) in patients with relapsed/refractory SCLC, LCNEC, and other relapsed/refractory epNECs1,2
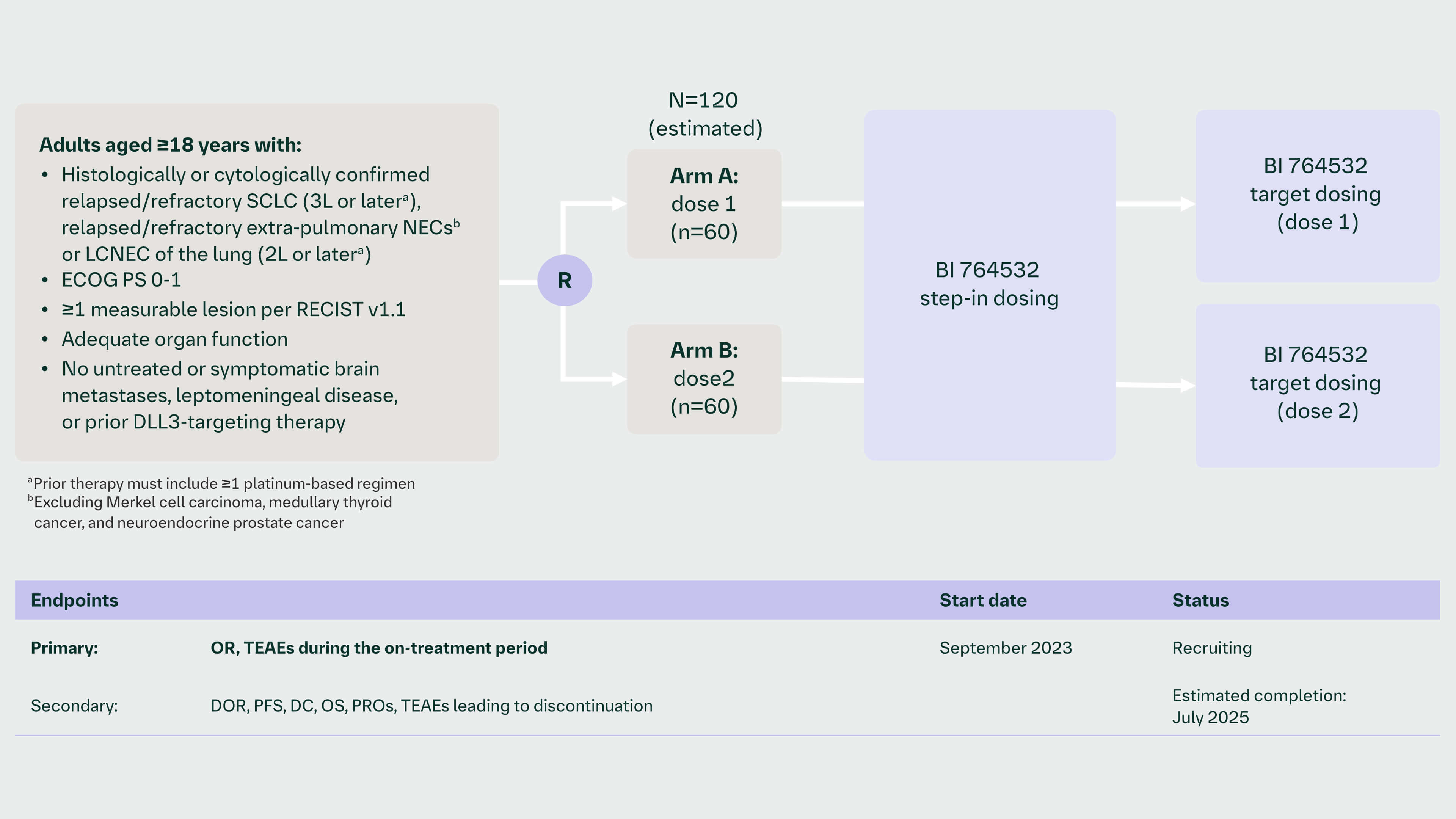
OR, objective response, DoR, duration of response, PFS, progression free survival, DC, disease control, OS, overall survival, PRO, patient reported outcome, TEAE, treatment emergent adverse events
1. Boehringer Ingelheim. Data on file; 2. NCT05882058. https://clinicaltrials.gov/ct2/show/NCT05882058. Accessed September 2023.