Clinical Results
Beamion LUNG-1 (1479-0001): Preliminary Phase I data
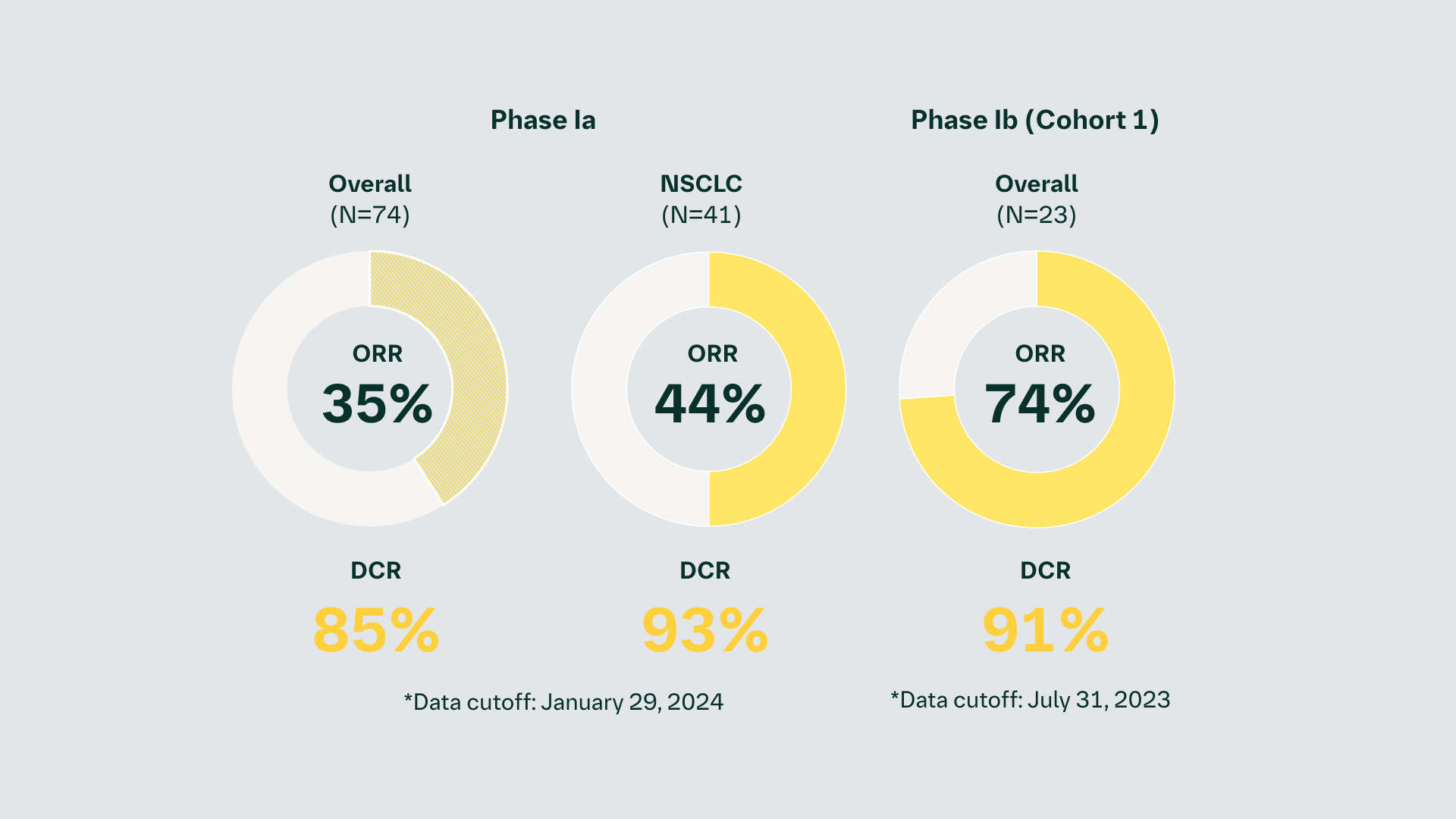
As of January 29, 2024, 83 patients have been treated in Phase Ia, while 42 patients have been treated in Phase Ib (Cohort 1) as of July 31, 2023
Preliminary safety data
In Phase Ia, MTD of zongertinib (BI 1810631) was not reached with either BID or QD schedule
Doses taken into dose optimization are 240 mg and 120 mg QD
Zongertinib was well tolerated with low rates of EGFR-associated AEs
In Phase 1a, the most common TRAEs occurring in >10% of patients were diarrhea (42.2%), rash (12%) and increased ALT and AST (8.4%)
In Phase Ib, 66.7% of patients experienced TRAEs, mostly grade 1 or 2. TRAEs occurring in >10% of patients were diarrhea (29%) and rash (21%)
The planned futility analysis in Phase Ib Cohort 1 was passed and the trial is continuing. Recruitment is ongoing for all cohorts
Heymach J, et al. NACLC 2023. OA01.28.